Zoonosis: Brucellosis in Animals and Humans: Difference between revisions
| Line 1: | Line 1: | ||
<!-- Do not edit this line-->{{Curated}} | <!-- Do not edit this line-->{{Curated}} | ||
==Introduction== | ==Introduction== | ||
<br>By Hannah Wedig<br><br> | <br>By Hannah Wedig<br><br> | ||
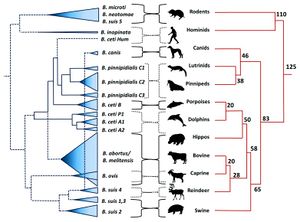
<br> [[Image: | <br> [[Image:BPPH.jpg|thumb|300px|right| <b>Figure 1.</b> The phylogeny of the genus <i>Brucella</i> aligned with the phylogeny of their respective, preferred host species. The width of the cones in the <i>Brucella</i> phylogeny is proportional to the number of strains analyzed for that species. The host phylogeny is represented in millions of years. <ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4026726/. Retrospective and prospective perspectives on zoonotic brucellosis. 2014. Moreno, E. <i>Front Microbiol.</i> 5: 213.]</ref>]] | ||
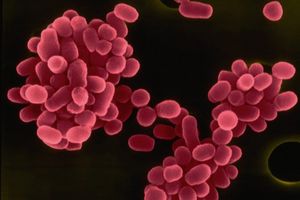
<br> [[Image:BM.jpg|thumb|300px|right| <b>Figure 2.</b> Colored scanning electron micrograph of <i>B. melitensis</i>. All <i>Brucella</i> species are non-flagellated, Gram-negative coccobacilli. <ref>[http://bioinfo.bisr.res.in/project/dofpath/images/bacteria/Brucella%20melitensis.jpg. "Brucella Melitensis." <i>Database of Bacterial Food Pathogen.</i> Birla Institute of Scientific Research, 2015.]</ref> | |||
<ref>[http://www.oie.int/doc/ged/D12397.PDF. Brucellosis in terrestrial wildlife. 2013. Godfroid, J., Garin-Bastuji, B., Saegerman, C., Blasco, J. M. <i>Rev. sci. tech. Off. int. Epiz.</i> 32 (1): 27-42]</ref>]] | |||
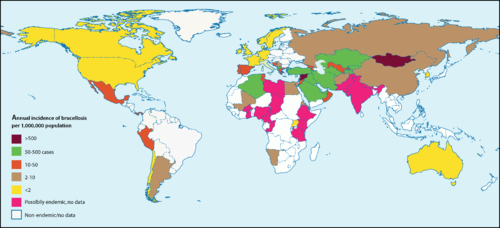
<br> [[Image: | <br> [[Image:bdist.png|thumb|500px|right|<b>Figure 3.</b> Global occurrences of Brucellosis in humans. The highest incidences are recorded in Syria (1603.4 incidences per 1M people per year), Mongolia (605.9 incidences per 1M people per year), and Kyrgyzstan (362.2 incidences per 1M people per year). The incidence of Brucellosis in the United States is 0.4 per 1M people per year. <ref>[http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0040317#pmed-0040317-g001 Ariza, J., Bosilkovski, M., Cascio, A., Colmenero, J. D. <i>et al.</i> "Perspectives for the Treatment of Brucellosis in the 21st Century: The Ioannina Recommendations." 2008. PLoS Med 4(12): e317]</ref>]] | ||
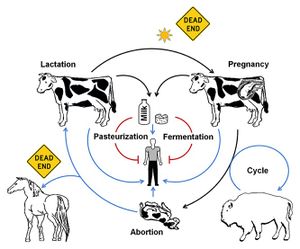
<br> [[Image: | <br> [[Image:BTP.jpg|thumb|300px|right| <b>Figure 4.</b> The various transmission pathways of <i>B. melitensis</i>. Pasteurization, fermentation, exposure to sunlight, and infection of certain paratenic hosts such as horses or humans are considered dead ends in that they either kill or prevent significant transmission of <i>Brucella</i>. <ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4026726/. Retrospective and prospective perspectives on zoonotic brucellosis. 2014. Moreno, E. <i>Front Microbiol.</i> 5: 213.]</ref>]] | ||
<br> [[Image:RBE.jpg|thumb|300px|right| <b>Figure 5.</b> Predictions for the development of vaccine-resistant <i>Brucella</i>. <b>(A)</b> The blue line represents the normal strain, the red line represents the resistant strain. The solid lines refer to the prevalence, the dotted lines refer to the basic reproductive rate. <b>(B)</b> Vaccines with lower than 75% efficacy pose a risk for the evolution of vaccine-resistant strains. <ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4026726/. Retrospective and prospective perspectives on zoonotic brucellosis. 2014. Moreno, E. <i>Front Microbiol.</i> 5: 213.]</ref>]] | <br> [[Image:RBE.jpg|thumb|300px|right| <b>Figure 5.</b> Predictions for the development of vaccine-resistant <i>Brucella</i>. <b>(A)</b> The blue line represents the normal strain, the red line represents the resistant strain. The solid lines refer to the prevalence, the dotted lines refer to the basic reproductive rate. <b>(B)</b> Vaccines with lower than 75% efficacy pose a risk for the evolution of vaccine-resistant strains. <ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4026726/. Retrospective and prospective perspectives on zoonotic brucellosis. 2014. Moreno, E. <i>Front Microbiol.</i> 5: 213.]</ref>]] | ||
Revision as of 15:51, 27 April 2017
Introduction
By Hannah Wedig
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
Sample citations: [7]
[8]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
Section 1
Include some current research, with at least one figure showing data.
Every point of information REQUIRES CITATION using the citation tool shown above.
Section 2
Include some current research, with at least one figure showing data.
Section 3
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ Retrospective and prospective perspectives on zoonotic brucellosis. 2014. Moreno, E. Front Microbiol. 5: 213.
- ↑ "Brucella Melitensis." Database of Bacterial Food Pathogen. Birla Institute of Scientific Research, 2015.
- ↑ Brucellosis in terrestrial wildlife. 2013. Godfroid, J., Garin-Bastuji, B., Saegerman, C., Blasco, J. M. Rev. sci. tech. Off. int. Epiz. 32 (1): 27-42
- ↑ Ariza, J., Bosilkovski, M., Cascio, A., Colmenero, J. D. et al. "Perspectives for the Treatment of Brucellosis in the 21st Century: The Ioannina Recommendations." 2008. PLoS Med 4(12): e317
- ↑ Retrospective and prospective perspectives on zoonotic brucellosis. 2014. Moreno, E. Front Microbiol. 5: 213.
- ↑ Retrospective and prospective perspectives on zoonotic brucellosis. 2014. Moreno, E. Front Microbiol. 5: 213.
- ↑ Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.