Estuaries
Introduction

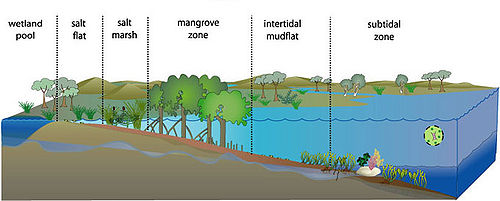
"An estuary is a partly enclosed coastal body of water with one or more rivers or streams flowing into it, and with a free connection to the open sea."[[3]].Estuaries are transition zones between rivers and the sea, which differ from both in abiotic and biotic factors [1]. They are also among the most highly productive ecosystems on the earth. The estuarine environment is characterized by a constant mixing of freshwater, saline seawater, and sediment, which is carried into the estuary from the sea and land. The mixture and fluctuation of salt and freshwater impose challenges to , the animals and microbes. Along the gradient of conditions from the open sea into the sheltered estuary the salinity ranges from full strength seawater to freshwater. Associated change is sedimentary conditions from fine sediment to coarse sediments. Other changes include nutrient input, pollutant and chemical concentration along with estuarine flows.
The activities of microorganisms dominate the functions and material cycling of estuarine ecosystems. Various nutrients flows dominated by microbial activities are processed in an estuary. Large numbers of bacteria, fungi and protozoa have been found in estuaries and benthic sediments. Their distribution, species abundances and activities interact with their physical and chemical environment.
The importance of estuary
The productivity and variety of estuarine habitats support a wonderful abundance and diversity of species. Thousands of species of fish, shore birds, marine mammals, clams, shellfish and other wildlife survive in and around estuarine habitats. Many fish and shellfish species, including most commercially and recreationally important species, depend on the sheltered waters of estuaries as home to spawn and for their offspring to grow and live. Due to the high productivity of living organisms, migratory birds also take estuaries as ideal places for resting and reproducing. In addition to serving as important habitats for wildlife, estuaries also provide valuable environmental services. The water flowing to the ocean carries sediments, organic and inorganic nutrients, and pollutants. Much of the sediments and pollutants are filtered out when they flow through wetlands, swamps and salt marshes. This filtration process deposits harmful pollutants and then creates an environment for microbial biodegradation of these sediments. Estuarine plants also can absorb tide and storm surges, providing peaceful and stable habitats for widelife. This natural buffer helps to prevent erosion and stabilize the coast. The transition character of estuaries provides important research value for scientists. A wide range of problems in biology, geology, chemistry, physics, and sociology are studied in and around estuaries. Estuaries also provide a great deal of aesthetic enjoyment for the people who live, work, or recreate in and around them. [[4]]
Physical environment
Physical characteristics
Water movementis the dominant controlling factor in estuarine ecosystem. The changes of physical factors occur quickly relative to biological and chemical transformations.
Circulation
Circulation is defined as the residual water movement, which is calculated based on different time scales. Circulation stimulates fluxes of dissolved constituents and particulate materials such as sediments, detritus, bacteria, and plankton. The energy driving estuarine circulation is from solar heating, gravitational attraction between the moon and the sun, and wind. A given estuary usually is dominated by one circulation type, but other modes of circulation can become predominant temporarily.[2]
Mixing events
In Estuaries, salt water mixes with water derived from land drainage. Mixing is the process whereby water is diluted or redistributed with other water body. Mixing events can be divided by long or short time scale. The estuarine circulation movements are the primary mechanism of mixing. Mixing changes the distribution in time and space of dissolved material in fresh and ocean water. The estuarine salinity alone beach is the most important indicator of mixing, that is, salinity can be used to track water source and mixing frequency.
Chemical environment
Salinity
Substantial river discharges and relatively shallow nearshore waters often result in large fluctuations and strong spatial gradients in salinity. In most estuaries, reduced salinity is associated with finer substrates, the finer substrate, the easier reduce salinity from estuaries.Salinity of estuaries usually increases away from a freshwater source such as a river, although evaporation sometimes causes the salinity at the head of the estuary to exceed seawater. The vertical salinity structure and the nature of salinity variation along the estuary are the features of the salinity structure of coastal waterways.[5]
Oxidation
Much of the organic matter carried to an estuary by rivers, produced by phytoplankton, or derived from marshes, is deposited on the sediment surface. Oxygen is the most important electron acceptor in organic matter respiration, but at the water column of anerobic estuarine or saturated sediment sulfate become more significant electron acceptors. The major product of sulfate reduction is hydrogen sulfide, which gives salt marsh soils a pungent smell. In general, the environment is oxidizing near the sediment–water interface and more reduced deeper in the sediment.
Autotrophic nutrients
Autotrophic nutrients are important for the functional estuarine ecosystems, because they are the raw materials for the primary producers. The concentrations of these nutrients change in estuaries due to the mixing of river and ocean water. Mcrobial heterotrophic activity and primary production play very important roles in the formation and turnover of organic matter in eutrophic estuaries. Higher microbial uptake and respiration rates happen when high organic nutrient input[2].
Biological interactions
Distribution of estuarine organisms
Plants and animals living in estuaries are mostly organisms with marine affinities that live in the central parts of estuaries. They either enter estuaries as part of a positive movement or migrate with water flows, or their ancestor move into estuaries and the offspring become residents in estuaries. True estuarine organisms could live in sea but are sometimes absent from the sea, probably due to competition from other animals. Migrant organisms spend part of their life in estuaries for feeding or reproducing. Others are purely migrants that use estuaries as routes to move, such as salmon and eels. Many studies of the distribution and abundance of animals and plants in estuaries have shown that the number of species within estuaries is less than the number of species within either the sea or the freshwater, but these species may reach very high abundances in estuarines [1].
Food web
Within the estuaries, the plants and other primary producers (algae) convert energy into living biological materials. Detritus feeders, plant grazers, and zooplankton are the primary consumers, and the secondary consumers and tertiary consumers include estuarine birds, ducks, invertebrate predators, and fish. Excreta and detritus pass to the decomposer tropic level where microorganisms break down the material. At each stage in this trophic sequence matter and energy are consumed, and some of it is excrete as waste, or converted into body growth or heat after respiration [1]. Movement through the food web is accompanied by the physical transportation of organic and inorganic. Study has shown that the primary producers and seston showed significant variations between dry and rainy season. Compositions of C and N in mixed zooplankton, copepods, filter-feeders bivalves and juvenile mullet were directly related with the seston signals [3].
Microbial communities
Bacteria
Bacteria are the most numerous organisms in the estuary, averaging between 10^6 to 10^7/ml organisms in water and 10^8 to 10^10 per dry weight of sediment. Sediments and salt marsh soil generally harbor more bacteria per unit volume than does the water column. Within the water column, high densities may be found in the surface layer than subsurface layer. Aerobic and facultative anaerobic bacteria are most common, and pseudomonads and Vibrio are the most often isolated species. Sediment and waterlogged soils show very high densities of bacteria, which decrease in abundant with depth of soils. Higher bacteria densities have been found in most estuaries than in nearby coastal seawater and river water [2].
Fungi
TThe number of fungi living in estuaries is extremely large. Some of fungi are unique in estuaries, while others have a broader range of habitats. Aquatic fungi and yeast dominate species in aquatic environment, few of fungi associate with particles or solid matters in the water. In sediments, the active species of fungi primarily are found in surface aerobic zones. The densities of fungi decrease rapidly with soil depth, but the spores of fungi are found throughout sediments [2].
Processes associated with microorganisms
Cycle of energy and matter in estuaries is closely related with microbial activity. It has been estimated that half of the aerobic and anaerobic transformations of organic matter in salt marsh are the result of microbial metabolism.
Carbon cycling
Bacteria show a variety of metabolic pathways related to carbon flow and cycling. Photosynthesis is mainly carried out by algae and phytoplankton in estuarine. Carbon fixing rate of phytoplankton shows marked seasonal fluctuations in hydrographic and nutrient parameters. As many of the sediment and water-logged soils of estuaries are anoxic, anaerobic decomposition is important. Complex organic matter is used by the fermenters and dissimilatory nitrogenous oxide reducers. The sulfate reducers and methane producers were once thought to have more restricted distributions [2]. Studies have shown seasonal and interannual dynamics of free-living bacterioplankton and labile organic carbon available to microbes along the salinity gradient of estuaries. Bacterioplankton abundance may be an important indicator of ecosystem health in eutrophied estuaries, because of the positive relationships between bacterioplankton abundance, microbially labile organic carbon (MLOC), and dissolved oxygen [4].
Nitrogen cycling
Nitrogen is a major limiting nutrient for primary production in estuaries. The N-cycling processes that are dominated by microbial activity include nitrification, dissimilatory nitrous oxide reduction, and nitrogen fixation. Nitrogen cycling in estuaries is related to the water mixing and microbial community dynamics. Nitrogen cycling across steep gradients in salinity, oxygen and dissolved inorganic nitrogen in sandy land and sea margin, coastal permeable sediments', it controls both the amount and form of nitrogen discharged to the coastal ocean. In one study, the abundance of betaproteobacterial ammonia-oxidizing bacteria (beta-AOB) was dramatically lower in the freshwater compared with saline stations, while ammonia-oxidizing archaea (AOA) abundance almost remained constant across estuarine sites. This differing response to salinity altered the ratio of beta-AOB to AOA. Analysis of ammonia-oxidizing enrichment cultures at a range of salinities revealed that AOA persisted solely in the freshwater enrichments [5].
Key Microorganisms

Several studies have described estuarine microbial diversity and how freshwater and marine microbial communities mix along estuarine gradients. Few reports have reported a unique estuarine bacterioplankton community. This is partly due to the dynamic nature of estuaries and the heavy influence on estuarine populations by those that wash in from adjacent environments. Most of the bacterioplankton in typical estuary are closely related to surrounding freshwater or marine bacterial groups and belong to the phyla Proteobacteria, Bacteroidetes, and Actinobacteria, with these estuarine phylotypes occurring within a range of salinity are considered as mixed freshwater or marine biota.these estuarine phylotypes occur within a range of mixed freshwater or marine biota [6]. It is therefore reasonable that similar shifts will occur in natural freshwater and marine microbial communities when they encounter estuarine gradients. Studies have shown that coastal communities were composed of typical marine populations and Proteobacteria phylotypes, including Roseobacter, and recently cultured Pelagibacter ubique and the Roseobacter isolate. Many of these estuarine phylotypes are most found in marine, some of these are typical freshwater-specific genotype, Alphaproteobacteria, Betaproteobacteria, Actinobacteria, and Verrucomicrobia, but they are relatively little overlap with the marine clades , suggesting that they are marine populations capable of adapting to estuarine conditions, including reduced salinity[7].
Cyanobacteria play an important role as primary producers, study in a pelagic of a shallow estuary found that Oscillatoriales and chroococcoid colonies dominated the cyanoplankton biomass, whereas Synechococcus-like Cyanobacteriacomprised 67.6–91.9% of the cyanobacterial biomass [8]. Methanogenic Archaea are important for the mineralization of organic matter in anoxic estuarine environments. Research in Beaulieu estuary shows Euryarchaeota, close related marine Archaeo and Methanosaeta phylotypes are high abundant, belonging either to the Methanosarcinales or the Methanomicrobiales orders. [9]. Sulfate-reducing bacteria often outcompete methanogens for hydrogen and acetate in estuarine sediments. In a meromictic lake sediment, sulfate-reducing bacteria were present in the entire water column, but the majority of them were present in the anoxic zone. C. phaeovibrioides, a green sulfur bacterium, was dominant at and below the chemocline [10].
Current Research
1. Ammonia-oxidizing archaea (AOA) are ubiquitous and abundant in marine waters and sediments, and they contribute to the N cycle in estuarine and coastal environments through coupled nitrification–denitrification or nitrification–anammox (anaerobic oxidation of ammonium) processes. Dang studied the sedimentary AOA diversity, amoA genotype communities and spatial distribution in the Changjiang Estuary and the adjacent East China Sea. Results indicated the gradients of surface-water salinity and sediment sorting coefficient are significantly correlated with the distribution of AOA communities. The archaeal amoA sequences had quite high similarity with known sequences from various soil environments or coastal and estuarine environments of the East Pacific Ocean, suggesting that similar AOA communities might exist in similar estuarine environments across broad geographical distances [11]. Caffrey studied the abundance of ammonia-oxidizing bacteria (AOB) and AOA amoA genes in six different estuaries at multiple sites. AOA, rather than AOB, are responsible for much of the nitrification in estuarine sediments. The potential nitrification rates increased as abundance of AOA amoA increased, suggesting that AOA are more significant than AOB in estuarine nitrogen cycling [12].
2.In bottom waters of stratified estuaries, oxygen consumed primarily by bacteria exceeded atmospheric and photosynthetic reoxygenation. This anoxic environment inhibited most living marine species, but a large number of bacteria and protists are still active by changing their metabolism to anaerobic respiration. The activity and phylogenetic composition of bacterioplankton communities across hypoxia/anoxia estuaries were studied. Bacterioplankton communities in anoxic estuaries of the Chesapeake Bay were very similar to those in oxic surface waters in summer even when oxygen respiration shifted to nitrate respiration, suggesting the microbes were adapted to a range of oxygen concentrations. . The forms of respiration used by bacterioplankton control redox conditions, which generate feedback to the phylogenetic composition of bacterioplankton communities ultimately. Estuaries are periodically refreshed with oxygen and chemical sediments from the ocean; thus, bacterioplankton communities shift their respiratory processes and phylogenetic composition as chemical conditions change seasonally [13].
3. The bioremediation potential of microbes in different environments is a hot topic for microbiologists. Some estuaries near urban and industrial areas received high inputs of a large variety of micro-pollutants including polycyclic aromatic hydrocarbons (PAHs). PAHs are toxic, mutagenic and carcinogenic for human health and the environment. Examination of the ecology of PAH degrading microorganisms is thus essential to prevent ecological damage caused by organic pollutants in estuary ecosystem. Research has found that a large number of bacterial species are able to bio-degrade PAHs, but the diversity of the bacterial community is also dramatically reduced due to special carbon source availability in PAH pollutants. Analysis of the 16S rRNA gene sequences confirmed that Cycloclasticus spp., plays a key role in degradation of low-molecular-weight PAHs in marine environments. Additionally, Pseudomonas spp., considered as a good PAH-degrading bacterial group in soil or in sediment, also increased their competition and adaptation in PAH degradation in a seawater macrocosm [14].
References
[1] McLusky, D.S. and Elliott, M. (2004) "The Estuarine Ecosystem: ecology, threats and management." New York: Oxford University Press Inc. ISBN 0-19-852508-7
[2] John W.DAY, Charles A.S, W.Michael K, Alejandro Y.A.(1989) "Estuarine Ecology." Wiley-Interscience; 1 edition. ISBN 0-10-0471062634.
[9] . Banning, N., Brock, F., Fry, J. C., Parkes, R. J., Hornibrook, E. R. C., & Weightman, A. J. (2005). Investigation of the methanogen population structure and activity in a brackish lake sediment. [Article. Environmental Microbiology, 7(7), 947-960.]
[10] . Ovreas, L., Forney, L., Daae, F. L., & Torsvik, V. (1997). Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. [Article. Applied and Environmental Microbiology, 63(9), 3367-3373.]
Edited by student of Angela Kent at the University of Illinois at Urbana-Champaign.