Symbiosis of Termites and the Microbes in their Gut: Digestion of Lignocellulose
The termite gut contains organisms from all three domains of life, Bacteria, Eukarya, and Archaea. (1) There is a great diversity of microbes in the termite gut, many of which are unidentified because of the tiny size of termites and also due to the challenge of growing them outside of the termite gut. Additionally many of the bacterial species are exclusively found in termite guts. (2) Termite digestion of lignocellulose is assisted by the microbes in their gut, and allows them to greatly contribute to the carbon cycle. (8)
Lower Termites versus Higher Termites
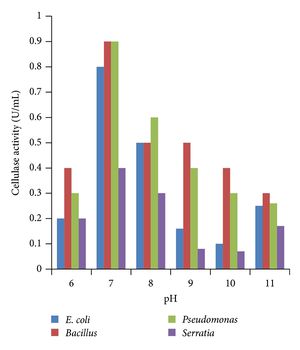
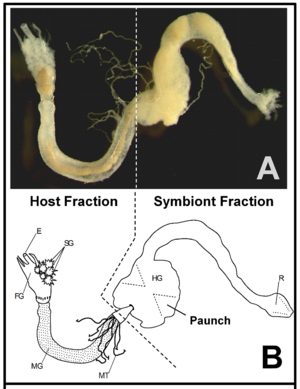
There is a distinction between lower termites and higher termites, mentioned throughout many studies of termite guts. Lower termites have many species of bacteria along with protozoa, while higher termites usually just have the bacteria and a more elaborate anatomy while lacking the protozoa. (3) Both higher and lower termites have microbes and enzymes in their hindgut, and this is therefore where the most symbiosis occurs. (Fig. 1) Bacteria and archaea are present in both types of termites, but cellulolytic flagellates are only found in lower termites. (8) The evolution of these flagellates has provided the termites with the ability to digest wood, and experiments have demonstrated that without these protists, lower termites starve. The dominant microbiota in lower termite hindguts are spirochaetes. (8) Higher termites do not have the protozoa that lower termites have in their hindguts that make cellulose digestion possible, so higher termites have evolved with different gut components and diets. (3) Some higher termites have a symbiosis with a fungus, Termitomyces, and Bacteroidetes and Firmicutes which thrive with a fungus-rich diet. Other higher termites that have cellulose-rich diets, have many spirochaetes and Fibrobacteres in their gut, as well as an anatomically evolved gut that has increased compartmentalization in the hindgut and increased alkalinity (more basic pH level) in anterior compartments. (8,9) It is possible that this high alkalinity could be the key to the mystery of how higher termites handle the degradation of lignin. (Figure 2)
Cellulase Activity
Termites degrade lignocellulose with the combination of their own “mechanical and enzymatic contributions” and the catalytic contribution of their symbiotic microbes. (8) Many studies on termite gut cellulose digestion have been conducted using carboxymethyl-cellulose as the substrate to measure cellulase activity in termites, but carboxymethyl-cellulose can be hydrolyzed in ways that aren’t specific to digestion in the gut, such as activity in salivary glands. Therefore, Tokuda et. al looked at the digestion of crystalline cellulose in termites and compared the different types of cellulase activity. (4) Their investigation found that most cellulose digestion occurs in the hindgut for termites that have flagellates, and the midgut for termites without the presence of flagellates in their gut. They also describe evidence of hingdgut bacteria containing 'cellulosome' complexes, explaining how they digest the food substrates with their cell wall by surrounding the food substrates. (4)
Metabolism
In lower termites, metabolic fermentation processes are credited to the flagellates, but similar to lignin degradation, less is known about the fermentation processes of higher termites. (9) The termite gut, in general, is not a simple anoxic environment, as it actually has a complex structure of different microenivronments. (5) As opposed to the bovine rumen and human colon, which only have anaerobic bacteria, the termite gut has the presence of oxygen and this effects the processes that occur. (6) For most lower and all higher termites, there is an accumulation of hydrogen in the hindgut paunches, suggesting it is a key metabolite. (8) Most of this hydrogen is used in homoacetogenesis and methanogenesis. (8) Part of what makes certain termite guts so interesting is the preference the bacteria have for acetogenesis over methanogenesis. (3) Termites that have fungi in their gut and feed on soil seem to perform methanogenesis more often than acetogenesis, but the higher termites that just feed on wood are the ones that prefer acetogenesis. (6) From what has been studied so far, formate is formed in the hindguts of many termites, which then either accumulates, oxidizes to carbon dioxide, or reduced to acetate. (8)

Lignocellulose Digestion
Lignocellulose digestion requires efficient cellulases and glycoside hydrolases to degrade the the cellulose and hemicellulose of the cell wall, but additionally a mechanism to handle the lignin barrier. This is accomplished through the combined effort of the host termite and its symbionts. (8) Both higher and lower termites have the enzymes needed for the first stage of the TCA cycle, but they lack an enzyme capable to convert pyruvate to acetyl CoA or acetate. (3) Hydrolysis of cellulose begins with endoglucanases released by the host: in lower termites the endoglucanases are secreted by the salivary glands, while they are secreted in the midgut epithelium in higher termites. (8) The flagellates in lower termites produce numerous exoglucanases, exoglucanases, B-glucosidases, and numerous other glycoside hydrolases. In higher termites, there are also many cellulases, xylanases and other glycoside hydrolases. Burnum et al. used metaproteomics to examine the hindgut of Nasutitermes corniger, a higher termite, and found almost a quarter of the 886 proteins they identified were enzymes and 36 were glycoside hydrolases. (7) Their results suggest these enzymes were important to the symbiotic relationship between the hindgut microbes and the termite host. He et al. studied Nasutitermes corniger as well as Amitermes wheeleri with the comparison of metaproteomic and metatranscriptomic analysis. (Fig. 3) Both N. corniger and A. wheeleri are higher termites, but they have different diets and habitats. (9) He et al. found different abundances of certain bacteria in the guts of the two termites, catering to their varied diets - cow dung for A. wheeleri and wood for N. corniger. In both, however, many glycoside hydrolases were found (similar to the observations of Burnum et al.) to assist in cellulose degradation. He et al. notes that there were no lignin degradation genes identified in either species’ hindguts. (9)
Further Research
It is still unclear how higher termites deal with lignin but He et al. suggest it could be the host’s own components, such as higher alkalinity in a certain part of the gut, or possibly unidentified microbes in a more aerobic component of the gut. This hole in the understanding of lignin degradation has not stopped people from researching termite gut symbiosis and how this could be used to convert lignified plant cell walls into soluble sugars. Brune et al. suggest that more knowledge on lignocellulose digestion in termites could inspire a more efficient production of biofuels in the future (8). Sethi et al. looked at three specific GHF7 (glucoside hydrolase) cellulases in the termite gut using cDNA sequences, PCR, and cellobiohydrolase activity assays. They found different levels of activity (GHF7-3 being the most active) which could be important for pursuing cellulases for biofuel production. (10)
References
(1) https://microbewiki.kenyon.edu/index.php/Termite_gut
(2) Hongoh, Yuichi. “Toward the Functional Analysis of Uncultivable, Symbiotic Microorganisms in the Termite Gut.” Cellular and Molecular Life Sciences 68, no. 8 (April 2011): 1311–1325. doi:10.1007/s00018-011-0648-z.
(3) Breznak, Ja, and A. Brune. “Role of Microorganisms in the Digestion of Lignocellulose by Termites.” Annual Review of Entomology 39 (1994): 453–487. doi:10.1146/annurev.en.39.010194.002321.
(4) Tokuda, G., N. Lo, and H. Watanabe. “Marked Variations in Patterns of Cellulase Activity Against Crystalline- Vs. Carboxymethyl-cellulose in the Digestive Systems of Diverse, Wood-feeding Termites.” Physiological Entomology 30, no. 4 (December 2005): 372–380. doi:10.1111/j.1365-3032.2005.00473.x.
(5) Ohkuma, M. “Termite Symbiotic Systems: Efficient Bio-recycling of Lignocellulose.” Applied Microbiology and Biotechnology 61, no. 1 (March 2003): 1–9. doi:10.1007/s00253-002-1189-z.
(6) Brune, A. “Termite Guts: The World’s Smallest Bioreactors.” Trends in Biotechnology 16, no. 1 (January 1998): 16–21. doi:10.1016/S0167-7799(97)01151-7.
(7) Burnum KE, Callister SJ, Nicora CD, Purvine SO, Hugenholtz P, Warnecke F, Scheffrahn RH, Smith RD, Lipton MS (2011) Proteome insights into the symbiotic relationship between a captive colony of Nasutitermes corniger and its hindgut microbiome. ISME J 5:161–164.
(8) Brune, Andreas. “Symbiotic Digestion of Lignocellulose in Termite Guts.” Nature Reviews. Microbiology 12, no. 3 (March 2014): 168–80. doi:10.1038/nrmicro3182.
(9) He, Shaomei, Natalia Ivanova, Edward Kirton, Martin Allgaier, Claudia Bergin, Rudolf H Scheffrahn, Nikos C Kyrpides, Falk Warnecke, Susannah G Tringe, and Philip Hugenholtz. “Comparative Metagenomic and Metatranscriptomic Analysis of Hindgut Paunch Microbiota in Wood- and Dung-feeding Higher Termites.” PloS One 8, no. 4 (2013): e61126. doi:10.1371/journal.pone.0061126.
(10) Sethi, Amit, Elena S Kovaleva, Jeffrey M Slack, Susan Brown, George W Buchman, and Michael E Scharf. “A GHF7 Cellulase from the Protist Symbiont Community of Reticulitermes Flavipes Enables More Efficient Lignocellulose Processing by Host Enzymes.” Archives of Insect Biochemistry and Physiology 84, no. 4 (December 2013): 175–93. doi:10.1002/arch.21135.
(11) Scharf, Michael E, Zachary J Karl, Amit Sethi, and Drion G Boucias. “Multiple Levels of Synergistic Collaboration in Termite Lignocellulose Digestion.” PloS One 6, no. 7 (2011): e21709. doi:10.1371/journal.pone.0021709.
Edited by Diana McDonnell, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.