Termite gut
Introduction
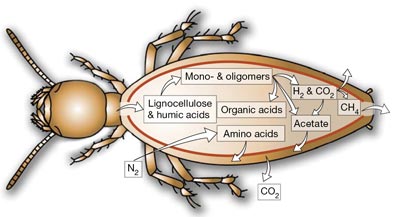
Termite has unusual characteristics of establishing themselves in lignocellulosic plant materials such as decaying wood, grass, animal dung or plant litter at various stages of humification. Despite their strange habitat, they play an important role as distributors of terrestrial decomposers of Earth’s major form of biomass. Termites are usually rich in carbon due to their diet; however, this also led to insufficient amounts of nitrogen. Facing this serious problem, many termites established symbiotic interactions with gut microbes to augment their nitrogen economy through recycling of uric acid and the acquisition of new nitrogen through N₂fixation.
Description of Niche
Location
Termite has unusual characteristics of establishing themselves in lignocellulosic plant materials such as “decaying wood, grass, animal dung or plant litter at various stages of humification.” (10) Despite their strange habitat, they play an important role as distributors of terrestrial decomposers of Earth’s major form of biomass. Termites are usually rich in carbon due to their diet; however, this also led to insufficient amounts of nitrogen. Facing this serious problem, many termites established symbiotic interactions with gut microbes to augment their nitrogen economy through recycling of “uric acid and the acquisition of new nitrogen through N2 fixation.” (11) Termite’s intestinal tract consists of three main parts: foregut, midgut, and hindgut. Foregut is composed of the crops and muscular gizzard, midgut is “a key site for secretion of digestive enzymes and for absorption of soluble nutrients,” and hindgut is “also a major site for digestion and for absorption of nutrients.” (12) Among these intestinal tracts, hindgut is known to be largest and have been thought of as “anaerobic digestors in which symbiotic gut microbes depolymerize cellulose and hemicellulose and ferment the resulting carbohydrates to short-chain fatty acids, which are then absorbed and oxidized by the host.” (10) Additionally, termite’s intestinal tracts comprise one or several dilated hindgut compartments and the volume of hindgut can be up to one-third of the body weight for phylogenetically low termites. It is said that termite gut resembles the rumen of sheep or cattle due to their “high concentrations of volatile fatty acids, the presence of fermenting bacteria and protozoa, and the occurrence of typical anaerobic activities such as homoacetogenesis and methanogenesis.” In the phylogenetically lower termites, anaerobic flagellates are present in a large fraction of hindgut volume. These flagellates phagocytize and degrade the wood particles comminuted by the termite. Nevertheless, a hylogenetically higher termite doesn’t compose of flagellates within their gut. (10)
Physical Conditions
Due to the small size of termites, analyzing termite guts have been very difficult without invasiveness techniques. Nonetheless, it became apparent that termites’ guts have a characteristic of being anoxic. “This characteristic becomes more apparent as one move from the foregut to the hindgut, and is greatest within microbe-packed portions of the hindgut.” Termites’ guts have anoxic conditions because of their steep oxygen gradients at the oxic-anoxic interface drive a continuous influx of O2 into the gut. Through examining termite guts with microsensor, it became evident that O2 may travel 50-200 um into the gut until it is used by the respiratory activity, creating a microoxic periphery around an anoxic center. “Radiotracer studies performed with the lower termite Reticulitermes flavipes have shown that the influx of O2 via the gut epithelium and its subsequent reduction in the hindgut periphery has a significant impact on carbon and electron flow within the hindgut microbial community.” (10) Although it became evident that termite gut has anoxic conditions, additional analyses were revealed by Bignell. According to his studies, microbe-filled paunch region of lower and higher termites yielded not completely anoxic conditions, but had a microoxic periphery. Through studies, Bignell and others indicated that pH and redox potential demonstrated significant transitions along the anterior to posterior axis of termite guts. However, the paunch region’s measurements were circumneutral and anoxic with a relatively low redox potential of -150 to -250mV. (3) The pH values of hindguts also showed neutrality: 6.2 to 7.6. (12)
This neutral pH value of hindgut can be altered if an environment changes. As some termites relies their diets on soil, soil-feeding termites’ hindgut pH value can be high as 11. (12) Soil-feeding termite exhibits extreme pH changes along the gut axis. Their anterior hindgut exhibits the most extreme alkalinity. This high pH transitions along the gut axis can be viewed as an evolutionary adaptation to “diet rich in tannins or other polyphenolic constituents” because it prevents precipitation of digestive enzymes and enhances the solubility of dietary proteins. As soil-feeding termite ingests soil organic matters, it needs to stabilize organic matters. Termite accomplishes this by alkaline extraction since alkaline in their gut separates organic nutrients from the organomineral aggregates. (13) These soil-feeding termites have other differentiations than wood-feeding termites. Although many of the phylogenetically higher termites are wood-feeding termites at various stages of humification and decay, more than half of all termites are soil-feeders. These humivorous termites’ gut is “highly compartmentalized and characterized by pronounced axial dynamics of the O2 and H2 partial pressure, and intestinal pH.” Due to these differences, production of H2, methanogenesis, and reductive acetogenesis by microbes in humivorous termites’ gut are unevenly distributed. (11) As these two different conditions establish differentiation among termites, other external factors can also distribute effects. For an example, as more and more H2 are added externally, methane emission rates of live termites are considerably stimulated. Despite the changes of emission rate of methane by H2, reduction of acetogensis from 14CO2 remains unaffected. (10)
Any adjacent community affects it? And do these conditions change or are they constant?
Many termites have close relation with tropical forest communities as they need woods and soils to acquire energies. As tropical forests contain numerous dry woods and plenty of soils, most of termites establish close relationship with them. Although many assume termites are voracious consumers of wood and they are correct, most termites have positive effects on tropical forests as they provide soil fertility. Additionally, their nests provide shelters and food for numerous numbers of associated organisms. Other communities that have significant affects on termites are microbes that live within them. Most of microbes establish themselves in termites’ gut and form symbiotical relationship with termites. They provide nitrogen and facilitate intake of numerous energies as termites can’t accomplish it alone. For an example, “the cellulolytic flagellates degrade cellulose to produce acetate, which is in turn absorbed by termites as their energy and carbon source.” (11) This symbiotic relationship of termite and microbes is constant because of their necessities from each other to survive. Without any individual, microbes and termites can’t acquire necessary nutrients in order to survive.
Who lives there?
Which microbes are present?
Termite gut exhibits one of the most complex microbial communities, consisting of diverse microorganisms from all three domains of life: Bacteria, Archaea, and Eukarya. By extracting DNA from the gut and comparing sequences of 16S rRNA genes with databases of rRNA sequences, predominant microorganisms in the gut community has been identified as well as their funcitons in the termite gut(1):
| Domain | Genus,Species | Brief Descriptions/Functions of Microbes |
| Bacteria | Treponema | Swim freely in the gut or attached to the protist; acetogenic, carry out acetogenesis |
| Bacteroides, Bacteroides termitidis | Fermentative, acidogenic; increase N source by recycling uric acid waste | |
| Desulfovibrio | Sulfate-reducing bacteria; transfer hydrogen as H₂ donor | |
| Citrobacter, Citrobacter freundii, Enterobacter, Enterobacter agglomerans | Nitrogen-fixing bacteria | |
| Enterococcus, Lactococcus | Lactic acid bacteria | |
| Archaea | Methanobrevibacter | Metanogens, associated with protists as symbionts; carry out metanogenesis and produce methane |
| Protists | Trichonympha, Mixotricha, Dinenympha, Euconomympha | Degrade endocytosed cellulose and produce H₂ plus CO₂. Anaerobic, occur on mitochondira in the cells |
Do the microbes that are present interact with each other?
Interaction between the microbes in the termite gut is highly mutual, usually beneficial for both microbes.
Prokaryotes are closely associated with protists as symbionts, either attached to the cell surfaces or live within the cytoplasm or nucleus of the protists. For instance, Treponema spirochete bacteria are attached to the special bracket-like structures on the plasma membrane of mixotricha and contributes to the movement of the host protist known as “motility symbiosis”(3). Treponema also benefits by living on and within the protist, easilly accessible to nutrients H₂ and CO₂ produced by mixotricha and utilize them to synthesize acetate and obtain energy for their own growth as well (5).
Another mutual relationship shown between Methanobrevibacter and parabasalids protist, H₂ plus CO₂ produced by protists also can be used by methanogens as energy source but they form methane,CH₄ in this case. Successful elimination of produced H₂ by endosymbiont’s H₂ evolution activity enables the protists to maintain optimal pH and stimulate its decomposition activity (4). These two groups of microorganism interact and work together to digest cellulose and enhance the cellulose fermentation.
Although most of the microbes act mutually, there is one exception between the relationship of methanogens and acetogens. Both take up H₂ and CO₂ as their substrates, thus they are likely to be in a compete relationship. Acetogenesis dominates methanogenesis from the same substrate, H₂ plus CO₂, because acetogenesis requires less energy loss of the termite by absorbing acetates but not methane as the energy source.(4)
Do the microbes change their environment?
The genus Treponema contains motile spirochetes that are embedded in the host’s cell membrane. They work as ectobionts to provide locomotion of the host cell by moving synchronizedly. Such relationship is known as the motility symbiosis (8).
Termites are on the nitrogen poor diet. Hence, the functional group of nitrogen fixers is essential to supplement a sufficient amount of nitrogen to the host protists. Treponema, Citobacter, Enterobacter, and Spirochaeta are some of the responsible nitrogen fixers. These microbes convert N2 from the atmosphere to NH3 and fix ~60% of the nitrogen supply of the host (7).
In the protest Pseudotrichonympha grassii, there are two gens enconding hydrogenosomal iron-hydrogenases. Two iron-hydrogenases are responsible for retaining optimal pH within the hydrogenosome in the protest cell. These enzymes catalyze H2 evolution instead of H2 uptake (6).
Do the microbes carry out any metabolism that affects their environment?
1. Nitrogen fixation and recycling of uric acid nitrogen
N₂ fixation is one of the crucial aspects of termite gut symbiosis, because termites feed on the nitrogen poor environment. The Nitrogen fixing bacteria such as Citrobacter freundii, Enterobbacter agglomerans, and Spirochaeta possess nitrogenase which reduces N₂ to 2NH₃, providing nitrogenous compounds that TG1 cannot synthesis. They play significant role in converting stable atmospheric nitrogen into something that host protists can uptake in order to supply the nitrogen need. Also, because the supply is so scarce, most of the nitrogen is recycled through the uric acid recycling to minimize the nitrogen loss. Generally, the required nitrogen fixation is low comparing to the high concentration in the termite due to the recycling of the uric acid (7).
2 .Acetogenesis/Methanogenesis
Both H₂/CO₂ acetogenisis and methanogenesis are anaerobic metabolism pathway that utilizes H₂ plus CO₂ generated by host protists during cellulose fermentation to synthesize acetate and methane, respectively. Prokaryotes associated with protists as symbionts are key microbes that are responsible for mediating these biosynthesis. Treponema acts as acetogens and carrys out acetogenesis by reducing CO₂: 4H₂ + 2CO₂ -> CH₃COOH + 2H₂O. Methanobrevibacter takes more of methanogenic role and also uses H₂ as its reducing agent for methane production: 4H₂ + CO₂ -> CH₄ + 2H₂O (3). Since the termite only can absorb acetate as its energy source, acetogenesis dominates methanogenesis in the termite gut to reduce energy loss as much as possible. Thus, only 1% of the carbon generated by microbes is lost in form of CH₄ and the rest 99% C source are emitted as CO₂ (3). But it appears to be that even small amount methane emitted by archaea-mediating metanogenesis are considered to be significant atmospheric methane source.
3. Cellulose degradation
Protists reside in the termite gut ingest wood particles in the form of cellulose and degrade it within their cells. Cellulolytic protists known as Trichonympha and mixotricha produce cellulases and various glycolytic enzymes that can break down cellulose and convert it into an intermediate product, malate (2). In addition, they carry specific anaerobic energy- generating organelle, hydrogenosome, where transferred malate from the cytoplasm is further fermented to produce CO₂, H₂, and acetate with the help of hydrogenase enzyme. During this fermentation process, ATP is also produced in the way and stored as energy available for both microbes and termite (2).
Current Research
The current research on the metagenomics is studying the DNA sequence of the termite gut to identify the microorganisms living in the termite which can be useful to produce the biofuel in the future. The identifying and studying microbes were the hardest part in the past, since only about 1 percent of the microbes were lab-cultivatable. However, the current fast and cheaper ways of gene sequencing methods have allowed microbiologists to study the other 99 percent. Scientist can extract the DNA from a certain sample, sequence that DNA, and derive the genomic clues to all microbes living in that certain environment. The microbes in the termite gut have ability to digest wood, such as converting cellulose into simple sugars that can be turned into ethanol by fermentation. Because “Termites are the world’s best bioconverters,” (9) the researchers are devoting their works to sequence the microbes to make the biofuel. Recently, researchers at the Joint Genome Institute have just finished sequencing the microbial community of the termite gut and have already identify a number of novel cellulases which breakdown cellulose into sugar. Their next goal is to adequately modify and use the microbes to synthesize an ideal energy producing organism (9).
References
(1) Ohkuma, M., and Kudo, T. “Phylogenetic Diversisity of the Intestinal Bacterial Community in the Termite Reticuliterrmes speratus”. Applied and Environmental Microbiology, p.461-468 (1996).
(2) Ohkuma, M. “Metabiolic Symbiosis between Termite Gut Protists and Their Intracellular Bacteria”. Noda Institute for Scientific Research, p.35-36 (2006).
(3) T. Abe, D.E. Bignell, and M. Higashi: Termites: Evolution, Sociality, Symbioses, Ecology. Kluwer Academic Publishers, Dordrecht, 2000. p.209-227., p.307-332
(4) Ohkuma, M. “Symbiosis within the gut microbial community of termites.” RIKEN Review No.41. Nov. 2001, p.69-72.
(5) Breznak, J. A. “Phylogenetic Diverisity and Physiology of Termite Gut Spirochetes.” Integ. And Comp. Biol, 42:313-318 (2002)
(6) Michael Pester and Andreas Brune. “Hydrogen is the central free intermediate during lignocellulose degradation by termite gut symbionts.” The ISME Journal (2007) 1, 551-565.
(7) John A. Breznak. “Phylogenetic Diversity and Physiology of Termite Gut Spirochetes.” Integ. And Comp. Biol., 42:313-318(2002)
(8) Claudia Husseneder, Billy R. Wise, and Dennis T. Higashiguchi. “Bugs in Bugs: The Microbial Diversity of the Termite Gut.” Proc. Hawaiian Entomol. Soc. (2007) 39:143-144
(9) Emily Singer. Why Termite Guts Could Bring Better Biofuels. Technology Review. Jan 17, 2007
(10) Brune, Andreas. “Microecology of the termite gut: structure and function on a Microscale.” Current Opinion in Microbiology 3:263-269 (2000)
(11) Brune, Andreas. “Termite guts: the world’s smallest bioreactors.” Tibtech 16 (1998)
(12) Breznak, John A. “Role of microorganisms in the digestion of lignocellulose by termite.” Annu. Rev. Entomol. 39:453-87 (1994)
(13) A. Kappler. “Influence of gut alkalinity and oxygen status on mobilization and size-class distribution of humic acids in the hindgut of soil-feeding termites.” Applied Soil Ecology 13:3 (1999)
(14)"Microbiologists find a New Source of Nitrogen Fixation" June 28, 2001 <http://www.nsf.gov/od/lpa/news/press/01/pr0154.htm>
(15) Termite Power. Biology at JGI <http://www.jgi.doe.gov/education/bioenergy/bioenergy_4.html>
Edited by Kevin Cho, Seyon Kim, Jee Shin, students of Rachel Larsen