Acanthamoeba polyphaga
Acanthamoeba polyphaga is a protozoa which has easily detectable genus classification due to its spiny projections but difficult to classify within the species level. It may infect individuals leading to infections such as Granulomatous Amebic Encephalitis (GAE) or Acanthamoeba keratitis
Introduction
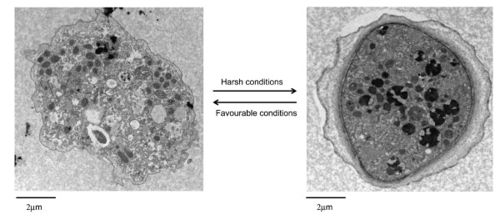
Environment
Acanthamoeba polyphaga is a free-living amoeba found in environments including soil, dust, air, seawater, tap water, and swimming pools [1].
Nutrition
A. polyphaga receives nutrition by consuming bacteria, algae, and yeast via phagocytosis. The amoeba ingests liquids through the process of pinocytosis. A. polyphaga acts as a secondary decomposer to remineralize soil with carbon, nitrogen, and phosphorous by consuming bacterial primary decomposers. Primary decomposers reduce organic materials into useful minerals. However, they are unable to release these minerals into the environment. A. polyphaga digests the primary decomposers and uses contractile vacuoles to release the environmentally useful bacterial nutrients from its food vacuole into the soil [1].
Lifecycle
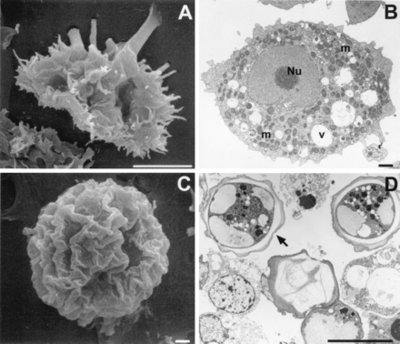
A. polyphaga, like the rest of the Acanthamoeba species, is divided into two lifecyles: the active trophozoite stage which reproduces via binary fission and the dormant, non-dividing cyst stage. Acanthamoeba polyphaga trophozoites contain one or more contractile vacuoles, lysosomes, digestive vacuoles, glycogen-containing vacuoles, a Golgi complex, smooth and rough endoplasmic reticulum, free ribosomes, microtubules, large numbers of mitochondria, and a single nucleus ([1], [2]). The cysts of A. polyphaga contain durable double walls for protection in harsh conditions. The trophozoite turns into a cyst under adverse environmental conditions such as during food deprivation, desiccation, and changes in temperature and pH [1].
Classification
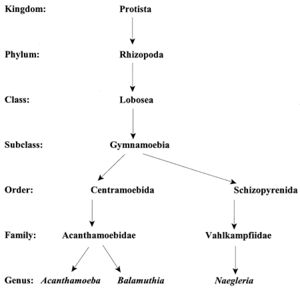
Current Classification
Acanthamoeba are termed amphizoic organisms due to their ability to exist as either free-living or parasitic. Identification at the genus level is easy to detect since all Acanthamoeba trophozoites contain spiny projections on the surfaces of their cell [1].
The species level has three morphological groups (I, II, and III) based on cyst size and shape. Species I have larger cysts, species II have a wrinkled outer cyst and a stellate inner cyst, and species III have a smooth ectocyst and a round endocyst. A. polyphaga is placed in the Species II group since it is characterized as having a wrinkled ectocyst and a stellate endocyst [1].
The accurate placement of species into one of the three group levels is difficult since cyst structure changes depending on environmental factors. Categorizing the species into immunological categories based on outward cell conditions is difficult since some species share antigenic or antibody determinants. The enzymatic patterns within the Acanthamoeba have been used to group individuals, but similarities exist between strains of separate species [1].
The Acanthamoeba phylogeny can be determined using sequence analysis of the 18s ribosomal RNA coding DNA (18s rDNA). Instead of separating Acanthamoeba into three different species, this analysis divides them into 12 groups based on sequence types. The genotypes are classified from T1 to T12 species. A. polyphaga is found in the genotype T4 group [3].
Granulomatous Amebic Encephalitis (GAE)
Granulomatous Amebic Encephalitis in Compromised Immune Systems
A. polyphaga can be pathogenic to humans with compromised immune systems and cause a rare but fatal nervous system disease called Granulomatous Amebic Encephalitis (GAE) [1]. The amoeba enters the body through the respiratory tract and invades the alveolar blood vessels, ultimately passing through the blood-brain barrier and entering the central nervous system (CNS) [2].
Diagnosis is difficult because symptoms of GAE are vague and resemble other common diseases. The symptoms include headache, fever, lethargy, stiff neck, and vomiting. A. polyphaga is not always detected in human serum and therefore cannot be detected when cultured. In severely immunocompromised patients, the brain lesions may not appear on magnetic resonance imaging (MRI) or on computerized tomography (CT scan). Thus, patients usually die before the protozoan is detected and identified. There is a greater than 90% mortality rate [2]. Currently, many patients are diagnosed during the autopsy by examination of brain tissue after death has already occurred [4].
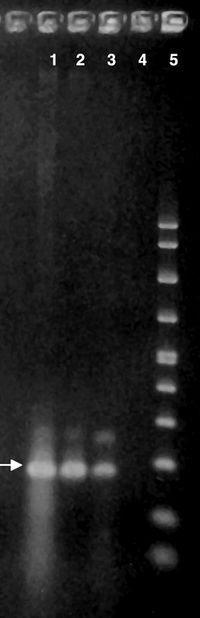
Detection Using PCR Gel
Recently, researchers have detected A. polyphaga DNA obtained from samples of cerebral spinal fluid by lumbar puncture. The samples of centrifuged cerebral spinal fluid in conjunction with Polymerase Chain Reaction (PCR) gel technique have revealed the presence of Acanthamoeba [4]. This discovery has lead to earlier detection and treatment options for immunocompromised patients suffering from Acanthamoeba infections.
In fact, a few patients have already benefitted from a new Acanthamoeba-specific PCR gel. A 25 year old patient with a combination of GAE, Milary Tuberculosis and Tuberculosis Meningitis was fortunate his Acanthamoeba was detected in the new gel. His illness was treated with the medication Miltefosine and the patient has been infection free for two years. However, detection in most patients is still rare since the amoeba cannot be cultured and usually cannot be isolated from cerebral spinal fluid [5].
Acanthamoeba keratitis
Acanthamoeba Keratitis in Healthy Immune Systems
Pathology can also occur in individuals with healthy immune systems. A. polyphaga can cause the ocular infection Acanthamoeba keratitis (AK). AK starts as a painful corneal infection that leads to breakdown of the cornea and surrounding tissues. If the infection is not treated immediately when the disease is limited to the cornea, the protozoan migrates into the inner eyeball leading to the loss of visual acuity, or blindness [1].
Disinfection Efficacies of Contact Lens Solutions
Improper use of soft contact lenses is the main cause of infection. When an individual wears soft contact lenses in the shower, hot tub, or swimming pool, the soft contact lens acts as a sponge and absorbs Acanthamoeba from the water and holds the microorganism against the cornea. A. polyphaga contains 130 kDa mannose-binding protein (MBP) located on the surface of the amoeba. The protein attaches to the cells of the cornea and produces a toxin that disintegrates the host cells into small particles which are digested by phagocytosis. The amoeba produces the enzyme plasmin to further degrade the basement membrane fibers under the epithelium [2].
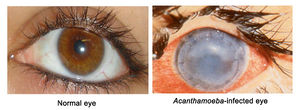
A clinical feature of AK is a cloudy cornea caused by infiltrating inflammatory cells such as neutrophils (white blood cells). Diagnosis can be difficult because AK is frequently mistaken as viral herpes simplex or a fungal infection [[1], [4]].
Acanthamoeba keratitis is also caused by contact lens wear for extended periods of time, lack of personal hygiene, and poor cleaning of contact lenses [2]. It is important to clean lenses with the proper contact cleansing solution because Acanthamoeba thrives in tap water [1]. However, when researchers tested the disinfection efficacies of 11 contact lens solutions, they found the A. polyphaga cyst was resistant to all contact lens solutions with the exception of only two brands available in the United States which contain hydrogen peroxide [6].
Conclusion
Acanthamoeba polyphaga’s role as a secondary decomposer that remineralizes soil by consuming primary decomposers and releasing nutrients should be studied further to enhance agricultural services. The cyst stage could provide low cost, long term benefits to farmers since cysts can withstand adverse environmental conditions and survive for many years. The amoeba could be used to produce nutrients for the soil to grow fruits and vegetables while at the same time keeping unwanted bacterial populations low. Thus, agriculturalists may consider harvesting the amoeba to help regulate soil conditions. Although the amoeba may benefit the soil, it can be a direct or indirect pathogen for humans.
A. polyphaga is a direct pathogen when it enters the respiratory tract and causes GAE or when contact lens wearers come in direct contact and contract Acanthamoeba keratitis. However, the amoeba can serve as an indirect pathogen as it becomes a host to other pathogenic organisms. For example, the easily destroyed pathogen that causes respiratory infections, Simkania negevenis, can hide and multiply within the amoebal cysts to survive long term under harsh environmental conditions. Thus, the amoebae may have an indirect role in the transmission of respiratory infections in humans [7].
Further research targeting specific Acanthamoeba species should help health care practitioners find new treatments specific to each group thus preventing diseases that are spread both directly and indirectly through A. polyphaga. In addition, the increased drug treatment specificity would lead to a decrease of unwanted drug side effects.
Due to the high prevalence of A. polyphaga in many diverse environmental settings, more public awareness is needed about general hygiene procedures to prevent disease. But since the amoeba is necessary for a healthy environment, the public should be educated about that aspect too.
References
Edited by Alexa Moy, a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.
