African Trypanosomiasis
Etiology
Taxonomy
| Domain = Protozoa | Phylum = Sarcomastigophora | Class = Zoomastigophora | Order = Kinetplastida | Family = Trypanosomatidae | Genus = Trypanosoma | Species = T. brucei
|NCBI: Taxonomy Genome: Genome|}
Description
[7]]]
African Trypanosomiasis also called Sleeping Sickness is caused by Trypanosoma brucei, a unicellular parasitic protozoan. There are two separate subspecies which cause sleeping sickness in humans, Trypanosoma brucei gambiense which accounts for around 98% of cases and Trypanosoma brucei rhodesiense which accounts for the remaining 2% of cases [1]. African trypanosomiasis is limited in epidemiology to areas of Sub-Saharan Africa [2]. Trypanosoma brucei was first described as the causative agent of Sleeping Sickness in 1901. Although by the mid-1960s the disease had been largely eliminated, the disease saw a reemergence countries in which the disease was endemic gained independence and their health systems subsequently collapsed [3]. The protozoan is spread via the tsetse fly, and key symptoms of the disease include headache, fever, stiffness and later coma and death [1].
Pathogenesis
Transmission
Sleeping sickness is most often transmitted via the bite of an infected tsetse fly, the most common and important vector of the parasite. When an infected fly bites a person, it transmits the parasite into the person after which the parasite will multiply rapidly within the subcutaneous layers of tissue [4]. Though rare it is possible for sleeping sickness to be transmitted other ways. The disease can be passed from mother to child when the parasite crosses the placenta and infects the fetus. The disease has also been documented to have occurred from the bite of infected blood sucking insects other than the tsetse fly. Most rare, but still documented are cases of sleeping sickness acquired due to laboratory accidents [1].
Infectious Dose, Incubation, and Colonization
The infectious dose of Trypanosoma brucei is at minimum between three hundred and five hundred microorganisms. Incubation period is different for the two different forms of the disease. In infections caused by T. brucei rhodesiense tend to have shorter incubation periods, with symptoms showing within one to two weeks of infection. For the gambiense strain, incubation periods can be longer, lasting several months before symptoms begin to show [5].
Epidemiology
Sleeping Sickness is limited to sub-Saharan Africa in its epidemiology. The two subspecies of the parasite which causes African Trypanosomiasis rhodesiense and gambiense are geographically separate from each other.
The rhodesiense-caused infection, often called East African Trypanosomiasis is present in specific areas within eastern and southern Africa. Countries with the highest prevalence of this form of the disease include Malawi, Zambia, Uganda, and Tanzania.
The gambiense-caused infection, which accounts for the vast majority of sleeping sickness cases, is also called West African Trypanosomiasis. The disease is found predominately in central and western African countries, most commonly in the Democratic Republic of Congo, Angola, Sudan, Chad, and the Central African Republic.
The disease very rarely occurs in any places outside of sub-Saharan Africa, and of those cases almost all occurred in people who had either recently traveled to areas in which the disease is endemic or recently immigrated from areas in which the disease is endemic [1].
Virulence Factors
Trypanosoma brucei has two major virulence factors. Perhaps the most important one is it's use of antigenic variation of its Variable Surface Glycoprotein (VSG) coat. Trypanosoma brucei is capable of using several mechanisms in order to make the VSG switches including both transcriptional switches, and DNA rearrangements. Antigenic variation is important because hosts can clear infections of Trypanosoma brucei using antibody-mediated immune response mechanisms. Antigenic variation however circumvents the ability of the host to recognize the parasite because it changes the composition and structure of the surface protein coat, forcing the immune system to respond to the parasite as if it is a completely new pathogen. Trypanosoma brucei have hundreds of VSG genes to switch between which aids in its ability to evade the immune response indefinitely [6].
The flagellum represents the second major virulence factor of this species. It is important for the organism's pathogenesis in human hosts as well as it's development within the tsetse fly vector. Trypanosoma brucei is an extracellular parasite and thus relies on its own motility to gain access to different tissues. The flagellum is what allows the organism to cross the blood brain barrier and cause the symptoms of late stage sleeping sickness. The flagellum is also important in host cell attachment. The flagellum produces outgrowths of membrane and extracellular matrix which allow it to form hemidesomosome-like plaques which attach the parasite to the host cell. This is particularly important in the colonization of the tsetse fly vector[7].
Clinical Features
Symptoms
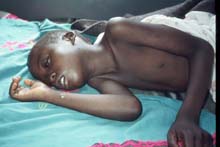
Sleeping sickness is characterized by two different stages. The beginning stage of the disease occurs while the parasite is still located only in the peripheral circulation. The disease progresses into the second stage when the parasite crosses the blood-brain barrier, infecting the central nervous system. Both of the subspecies which cause African Trypanosomiasis have different progressions and symptoms but ultimately if left untreated both result in coma followed by death [1].
Trypanosoma brucei rhodesiense which causes close to just 2% of all human sleeping sickness cases is characterized by rapid progression. The most common symptoms include headache, fever, joint aches and stiffness, and enlarged lymph nodes. Patients may also develop a large sore at the site of the tsetse fly bite. All of these symptoms usually appear within one to two weeks following infection with the parasite. It takes only several weeks for the parasite to cross the blood-brain barrier and infect the central nervous system. After infection by this subspecies, if left untreated, death typically occurs in just several months [1].
By comparison progression of sleeping sickness caused by the gambiense subspecies is much slower and initial symptoms of the disease are similar to that caused by the rhodesiense subspecies but much milder [2]. Weight loss and general fatigue are also common symptoms associated with infection by this subspecies. With this infection it typically takes somewhere between one and two years for the parasite to cross the blood-brain barrier, however it may take longer. When untreated death occurs most often within six years and infection rarely lasts longer than six to seven years [1].
Several characteristic symptoms occur when the parasite (either subspecies) crosses the blood-brain barrier and infects the central nervous system. The sleep cycle is almost always disturbed, with patients experiencing daytime sleepiness and issues sleeping during the night. Personality changes and confusion are also apparent once the infection reaches the CNS. Furthermore patients may experience partial paralysis and difficulties with balance. When left untreated the disease will progress eventually causing frequent seizures, severe somnolence, coma and multi-system organ failure, inevitably leading to death [1].
Morbidity/Mortality
Left untreated sleeping sickness is always fatal. The majority of deaths from sleeping sickness, around eighty percent occur within the first six months of infection. However the mortality rate of sleeping sickness when diagnosed within the first stage and treated is only about six percent [8].
Diagnosis
Diagnosis is dependent on a series of exhaustive screening tests. There are three steps in the process. Patients are first screened for potential infection using serological tests combined with an examination to check for any clinical signs. The serological tests are effective only in identifying the gambiense infection [2]. Next it is determined whether the parasite is present in the patient, and if so which subspecies it is. Finally the disease progress must be staged in order to determine treatment options. Staging is done by performing a lumbar puncture to obtain and study samples of the patient’s cerebrospinal fluid. CSF is also used to determine a general prognosis of the particular infection [2].
Early diagnosis of African Trypanosomiasis is critical because of difficulties with late stage disease treatments. Furthermore because the gambiense infection can have a very long asymptomatic phase, it is recommended that in areas of particular risk, the population is screened regularly for sleeping sickness to promote early diagnosis and reduce the risk of transmission [1].
The exhaustive and labor intensive procedures involved in diagnosing sleeping sickness can pose problems. Resources needed for diagnosis, including both health care workers and materials, are often scarce in the regions of Africa in which sleeping sickness is endemic. It is because of this that many people continue to die of the disease before they can ever be diagnosed or begin treatment [9].
Treatment
Treatment for African Trypanosomiasis is dependent both on the form of the disease and its staging. Early detection is very important for treatment of this disease because the drugs which can be utilized during the first stage of infection have a much lower toxicity to the patient and are easier to administer. Furthermore early detection increases the prospect of a full recovery from the disease [1].
There are two widely used first stage treatments for sleeping sickness. Pentamidine is used for treatment of the T. brucei gambiense infections. It was first discovered in 1941. Its side effects are generally mild and most people have little problems tolerating the drug, however some significant adverse side effects have been documented including low blood glucose, hypotension, and nephrotoxicity. For the treatment of the T. brucei rhodesiense in the first stage, the drug Suramin is used. It was first discovered in 1921. This drug has more significant adverse effects than those of Pentamidine and can include neuropathy, fatigue, anemia, rash and hyperglycaemia [2].
Second stage treatment is more difficult and in many ways more dangerous. Melarsoprol which was discovered in 1949 is used to treat both forms of sleeping sickness. This drug is derived from arsenic and as expected can be very dangerous. There are several very dangerous side effects including convulsions, loss of consciousness, and encephalopathy. Encephalopathy is the most worrisome side effects as it can be fatal. It occurs in about five to ten percent of patients treated with Melarsoprol [10]. Cases of sleeping sickness resistant to Melarsoprol have been documented recently, primarily in the region of central Africa. Another drug, Eflornithine, discovered in 1990 is used to treat gambiense-caused infections. It is less toxic than Melarsoprol, however the dosage and regimen is long and difficult, often making it impractical for use in many cases. Another drug marketed for use in American Trypanosomiasis called Nifurtimox was proposed in 2009 to be used in conjunction with Eflornithine for the treatment of gambiense-caused African Trypanosomiasis. After efficacy and safety trials, it has been approved as an option for treatment [2].
Prevention
To date there are no vaccines effective against either form of African Trypanosomiasis, nor are there any drugs which are recommended for use as prophylactic treatment in areas where there is a high risk of infection. Currently the only forms of prevention and control of the disease are aimed at preventing tsetse fly bites. There are several methods which can reduce the risk of being bitten by a tsetse fly. For example, wearing long sleeves and pants of medium weight material can protect the arms and legs from bites. Clothing which blends in with the environment may also help prevent bites since flies are attracted to bright and very dark colors. Avoiding bushes may also help, particularly in the middle of the day. Tsetse flies are not usually active at the hottest part of the day, and tend to gravitate toward the shade which bushes provide. Further it is important to be aware of your surroundings. Tsetse flies are often attracted to the motion of and dust disturbed by moving vehicles, so one should exert caution when getting into cars while in high risk regions [1].
Host Immune Response
Because Trypanosoma brucei has such an incredible ability to evade the host immune system, the response which the host mounts against the parasite is minimal at best. Rather the trypanosomes evade detection by using consecutive rounds of antigenic variation by switching out VSG gene. Studies have been done regarding complement pathway detection in infections of Human African Trypanosomiasis. It has been determined that alternative pathway is triggered completely and the parasite is lysed only if the parasite is not coated by VSG. When the parasite is coated with VSG however, complete lysis is prevented. In brucei species which infect humans, VSG almost always coats the parasite, thus blocking lysis of the pathogen [11].
References
1 Center for Disease Control and Prevention (CDC). African Trypanosomiasis (also known as Sleeping Sickness). Parasites. http://www.cdc.gov/parasites/sleepingsickness/disease.html
2 World Health Organization (WHO). Human African Trypanosomiasis. African Trypanosomiasis. http://www.who.int/trypanosomiasis_african/en/
3 Simarro, Pere P., Diarra, Abdoulaye, Ruiz Prostigo, Jose A., Franco, Jose R., Jannin, Jean G.. The Human African Trypanosomiasis Control and Surveillance Programme of the World Health Organization 2000-2009: The Way Forward. Public Library of Science. http://www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0001007
4 The Editors of Encyclopedia Britannica. Sleeping Sickness. Encyclopedia Britannica. http://www.britannica.com/EBchecked/topic/548611/sleeping-sickness
5 Despommier, Dickson D., Gwadz, Robert W., Hotez, Peter J., Knirsch, Charles A.. Summary of Basic Science and Clinical Information for African Trypanosomiasis. Parasitic Diseases, 5th Edition. http://www.medicalecology.org/diseases/d_african_trypano.htm#sect4
6 Aitcheson, Niall, Talbot, Suzanne, Rudenko, Gloria. VSG Switching in Trypanosoma brucei: antigenic variation analyzed using RNAi in the absence of immune selection. Mol Microbiol. 57(6): 1608-1622. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1618954/
7 Ralston, Katherine S., Kabututu, Zakayi P., Hill, Kent L. et al. The Trypanosoma brucei Flagellum: Moving Parasites in New Directions. Annu Rev Microbiol. 2009; 63(10). http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3821760/#!po=1.08696.
8 Odiit M., Kansiime F., Enyaru JC. Duration of symptoms and case fatality of sleeping sickness caused by Trypanosoma brucei rhodesiense in Tororo, Uganda. East Afr Med J. 1997. Dec; 74(12): 792-5. http://www.ncbi.nlm.nih.gov/pubmed/9557424.
9 Chappuis, Francois, Loutan, Louis, Simarro, Pere, Lejon, Veerle, Buscher, Philippe. Options for Field Diagnosis of Human African Trypanosomiasis. Clin. Microbiol. Rev. 2005. 18(1):133-146. http://cmr.asm.org/content/18/1/133.full.
10 Barrett M P, Boykin D W, Tidwell R R, et al. Human African trypanosomiasis: pharmacological reengagement with a neglected disease. Br J Pharmacol. 2007. 152(8): 1155-1171. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2441931/.
11 Vincendeau, Philippe and Bouteille, Bernard. Immunology and immunopatholgy of African trypanosomiasis. An. Acad. Bras. Cienc. 2006. 78(4). http://www.scielo.br/scielo.php?pid=S0001-37652006000400004&script=sci_arttext
Created by Madeline Gabe, student of Tyrrell Conway at the University of Oklahoma.