Alcaligenes Eutrophus and Its Role in Biodegradable Polymer Production
Introduction
By. Angel Mogie
General
Alcaligenes eutrophus (sometimes referred as Ralstonia metallidurans, Figure 1) is a gram negative bacillus with an optimum-growth temperature of 30 ̊C [1]. It is a non-spore forming, obligate aerobic, and facultatively chemolithoautothropic bacterium that can thrive in environments containing millimolar concentrations of some toxic heavy metals such as Zinc, Cadmium, Cobalt, Lead, Mercury, Nickle, and Chromium [2]. In the presence of nitrate, A. eutrhopus can grow anaerobically. It naturally synthesizes polyhydroxybutyrate (PHB), a specific type of polyhydroxyalkanoates (PHA) that is used in the manufacture of biodegradable plastic.
The Genes Encoding the Enzymes: phbC-phbA-phbB
There are three regulatory enzymes involved in the bioproduction of PHB in Alcaligenes eutrophus: 3-ketothiolase, acetoacetyl-CoA reductase, and PHA syntase, encoded by the genes phbA, phbB, and phbC respectively. The molecular genetics and mapping of these enzymes were done in E. coli using the A. eutrophus strains of H16, 11599, 1159981, PHB 2, PHB3, PHB19 [3].
In 1989, Sinskey and Peoples identified the location of phbA and phbB genes on plasmid, a single helix double-stranded loop DNA that can replicate independently of the chromosomal DNA, pAeT29 of E. coli and found that their expressions were under the control of Alcaligenes eutrophus promoter. Later in the same year, the two researchers mapped the location of phbC— the phbC gene is located upstream from phbA-phbB (Figure 2). Additionally, they also observed reduced activities in both thiolase and reeducate. Their results suggest that in PHB2 and PHB3 strains, all three genes are expressed from the same promoter located upstream from phbC
Moreover, Sinskey and Peoples also proposed that Alcaligenes eutrophus had at least two P-ketothiolase and acetoace- tyl-CoA reductase enzymes. Thiolase and reductase enzymes, when they interact with either enoyl-CoA hydratase or epimerase or both, can lead to the formation of D(-)-3-hydroxybutyryl-CoA. In E.coli the expression of phbC alone results in a diminished amount of PHB synthesis and an insignificant rate of PHB polymerase activity. The absence of both phbA and phB genes of A. eutrophus leads to no synthesis of D(-)-3-hydroxybutyryl-CoA. Only when the three A. eutrophus genes, phbC-phbA-phbB, were present in E. coli, then greater than 50% of PHB production was observed. They also figured that PHB production was inhibited by the presence of nitrogen. Moreover, they proposed that the interaction of thiolase and/ or reductase with the polymerase was necessary for the polymerase to function.
Heavy Metals Resistance
Among the many hydrogen-oxidizing bacteria, the Alcaligenes are characterized by their ability in forming two hydrogenases: an NAD-reducing hydrogenase found in the cytoplasm and a membrane-bound hydrogenase [2]. The hydrogen-oxidizing trait of A. eutrophus is encoded by a 450 kb plasmid, which was identified through a plasmid transfer to Hox- cells lacking the plasmid. Two genes encoding the heavy-metal resistance are located within two megaplasmids (pMOL28=180 kbp and pMOL30=240 kbp). These two megaplasmids also carry genes that express cation-efflux pumps for both bacterial inner and outer membranes.
PHB
Discovery
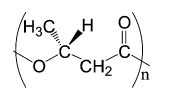
Maurice Lemoigne (mid-1920s) was the first person who discovered monopolyester of identified poly(3-hydroxybutyrate) or PHB (Figure 3) [4]. It took a while until the discovery was made known to public because it was originally written in French. Later on, PHB was chemically identified by Staudinger (1920s). However, PHB did not get the same level of attention from the public and scientists as prototypical biodegradable thermoplastic until thirty years later. The Imperial Chemical Industries Ltd. (1980s) was the first to do intensive studies on PHB in multiple research areas, including genetic engineering, biotechnology, and the enzymes responsible for biosynthesis and biodegradation. PHB is a reverse polymer found in bacteria and it can produce both the monomer and polymer of [R]-3-hydroxybutyric acid
Synthesis in Alcaligenes eutrophus
In A. eutrophus, the synthesis of PHB involves glucose as a carbon source and three enzymes: 3-ketothiolase, acetoacetyl-CoA reductase, and PHA synthase [5]. The first enzyme, 3-ketothiolase, is responsible for carrying the condensation reaction of acetyl-CoA into acetoacetyl-CoA. Then, acetoacetyl-CoA reductase reduces acetoacetyl-CoA into R(-)-3-hydroxybutynl-CoA, with NADPH serving as the reducing agent. R(-)-3-hydroxybutynl-CoA is then polymerized into PHB with the aid of PHA synthase (Figure 4). A. eutrophus can produce an amount of PHB equal to 80% of their dry body weight when grown in sugar-rich but nitrogen/ phosphate-deprived media.
Alcaligenes eutrophus synthesizes different kind of polymers based on the carbon source it is exposed to in the media. For example, having propionic acid instead of glucose as the main carbon source leads to the production of a polymer containing 3-hydroxybutyrate a (3HB) and 3-hydroxyvalerate (3HV) instead of PHB (Fig. 4). On the other hand, completely substituting glucose with valeric acid leads to higher production of polymer in A. eutrophus— euqal to 90% of the dry body weight.
Physical Characteristics: Benefits and disadvantages
PHB is a rigid homopolymer and is a fragile thermoplastic. It has 100% stereospecificity with the asymmetric D(-) molecule and therefore is highly crystalline[4]. Unlike many other biodegradable polymers, PHB is resistant to moisture, impermeable to oxygen, and insoluble in water, which makes it particularly beneficial for the food packaging industry. However, PHB’s melting point (175 ̊C) is very close to its degrading temperature (185 ̊C ), which is why it is so fragile and hence hard to mold [5]. The fragility of PHB can be lowered by integrating co-monomer 3-hydroxyvalerate or mixing PHB with more elastic polymer. The PHB copolymer containing 3-hydroxyvalerate unit P(3HB-co-3HV) is one of the developed forms of PHB with better mechanical functions: the more 3-hydroxyvalerate unit makes PHB tougher, more elastic, increases its elongation to break, and lowers its melting point but does not change its degrading temperature. Hence, the physical properties of PHB can be controlled by the amount of integrated 3-hydroxyvalerate during fermentation.
How is PHB Biodegradable?
Alcaligenes eutrophus forms PHB as a way of fixing carbon and as a form of intercellular storage [4]. When the bacterium runs out of extracellular carbon, PHB is broken down effectively and quickly into organic compounds to be reused. In fact, A. eutrophus is not the only bacterium that degrades PHB.
Lemoigne (1923-1927) discovered that B. megaterium releases [R]-3-hydroxybutyric
in an aqueous environment. Wilkinson et al. (1958) also studied B. megaterium and figured out that this bacterium degrades PHB and releases both acetoacetic acid and acetic acid as byproducts. In 1962, Merrick et al., worked on B. rubrum and found that the bacterium self-catabolizes its “native” granules through hydrolysis with the aid of the enzyme depolymerase (sometimes referred as hydrolase). This was followed by Williamson et al., in 1967, who discovered a specific dehydrogenase that convertes [R]-3-hydroxybutyric acid to acetoacetic acid. Another enzyme that carries the synthesis of acetic acid from acetoacetic acid was classified by Dawes and his team in 1973.
Base on those discoveries above, the biodegradation of PHB into simple organic compounds can be done through any of these possible ways:
• PHB can be broken down through hydrolisis with the aid of depolymerase—an enzyme secreted by many bacteria and fungi. In 1965, Delafield, Doudoroff and co-workers identified some pseudomonas that treat PHB as their energy and carbon source.
• Many microorganisms have the ability to both catabolize PHB and metabolize [R]-3-hydroxybutyric acid.
• Depolymerases are associated with long alkyl chained PHA classes.
• Polyester depolymerases are found in many different organisms and their characteristics and structure have been well studied.
Also, as Lee (1996) argues, human blood plasma has the ability to catabolize PHB and a fairly high concentration of the degradation byproduct has been detected. Hence, it is strongly conceivable that PHB is not poisonous to either plants, mammals, or the environment.
Environmentally vs. Economically friendly plastics
In order to be cost efficient, it is crucial to produce polymers using methods that will yield high productivity as well as lower cost. Amongst the many biodegradable polymers, PHB, poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and poly (3-hydroxyhexanoate-co-3-hydroxyoctanoate) are the ones that have been produced with high productivity [6]. In order to reduce the production cost, a gene transfer from A. eutrhopus to the plant Arabidopsis thaliana (Figure 5) can be done through genetic engineering.
Since A. eutrhopus belongs to the kingdom of prokaryotes, its DNA is not enclosed by a nucleus, and therefore its genetic information, as a single stranded plasmid, can be transferred to other organisms [7]. The three genes of A. eutrhopus, which are responsible for the expression of enzymes involving in the manufacturing of polymer, can be inserted into the DNA of A. thaliana through a technique called gene-splicing. Plastic polymers were found in the cytoplasm, nucleus, and vacuole of the plant and harvested through multiple-step chloroform extractions to separate the polymer from plant materials. Extracting polymer directly from A. eutrhopus could cost $4 per pound while harvesting this plastic from A. thaliana would only cost about $1.50 per pound. However, although it is less costly to produce polymer by transgenic plant, A. thaliana only yields polymer equal to about 14% of its dry weight. More studies on how to improve productivity is needed in order for it to be economically significant to the biodegradable polymer production [6]. However, neither the A. eutrhopus nor the A. thaliana means of harvesting polymer costs nearly as little as “normal” petroleum-based plastic production, which only costs about 50 cents per pound [7]. Per year, approximately 600 tons of biodegradable plastic are produced while one factory produces 100,000 tons on average of non-biodegradable plastic.
Potatoes are also another candidate for genetic engineering with the goal being to replace starch production with polymer production. Another group of plant polymer-producer candidates are oilseed crops. Yet further studies on how to develop polymer synthesis in plants are definitely needed [8].
Environmentally and Economically friendly: Recombinant E. coli in PHB Production
One of the major downsides of biodegradable plastic is its much more expensive cost of production compared to the much lower budget petroleum-based plastic fabrication. Although it is healthier for the Earth, PHA needs to be manufactured in a more cost-friendly way in order to be more marketable than the “bad” plastic. One of the alternatives in lowering the production cost of PHB is through fermentation reaction involving bacteria other than A. eutrophus. There are several reasons why harvesting PHB from A. eutrophus would cost more than if it were harvested from other bacteria, for example the lab-friendly E. coli. There are three major aspects that determine the efficiency of PHB production [9]:
1) synthesis by the producer base on the carbon source
2) the rate production and accumulation of PHB
3) the percent recovery and level of purity.
The third factor, percent recovery and purity, is usually the driving force that determines how big the production cost is. Under the same treatment, more PHB is recovered from recombinant E. coli compared to A. eutrophus. Furthermore, PHB produced by recombinant E. coli has 95% purity and 96% recovery— about 16% higher than the 80% recovery of A. eutrophus-produced PHB [10].
The recovery of PHB in E. coli can be done by having one of its strains, XL1-Blue, recombinantly harboring the genes of A. eutrophus necessary in PHB biosynthesis (pSYL 104) [10]. On top of the higher recovery and purity, PHB recovered from E. coli has granules with notably larger diameter, 1.13-1.25 um, than the PHB granules extracted from A. eutrophus which are only 0.1-0.8 um. E.coli can also utilize wider range of susbtrates e.g. lactose, sucrose, xylose, etc. Production time is much shorter in E. coli (24 hours) compared to A. eutrophus (three days). Moreover, in recombinant E. coli, plydispersity can be controlled by manipulating the activity of synthase. However, A. eutrophus produced PHB is more moldable than that produced by E. coli with its highly crystalline morphology.
Purity
Besides its high stability against molecular catabolism, recombinant E. coli PHB is also more digestible by its PHB-recovery treatment, hypochlorite, than A. eutrophus PHB [10]. These two characteristics are the major reasons for the high purity of recombinant E. coli PHB. PHB recovery using 20% sodium hypochlorite results in 88% purity of A. eutrophus PHB and 93% purity of E. coli PHB (Figure 6).
PHB from recombinant E. coli does not get hydrolyzed during or after fermentation due to its lack of intracellular PHB depolymerase. This is another advantageous characteristic of E. coli PHB that is absent in A. eutrophus PHB. In the recovery step involving SDS as treatment, the conditions have to be heated to 80 ̊C or above for the cells to be digested and to avoid nucleic acid suspension. If the temperature is not high enough during recovery, PHB cannot be harvested from the A. eutrophus since the lysate will rigorously aggregate. In E. coli, heat treatment is not needed due to the special characteristics mentioned earlier. This also leads to a higher percentage of PHB purity.
The difference in genetic information might be the basis of the difference between performances in PHB purity in A. eutrophus since and E. coli. Some observed phenomena that explain the high recovery of E. coli PHB are: 1) some PHB granules are large enough and hence increases stability, and 2) the more the PHB granules, the weaker the E. coli cell wall becomes and hence the host organism is easier to degrade.
Recovery
Recovery is the crucial step of PHB production since it could make the production either economically efficient or inefficient. One of the commonly used recovery treatment is sodium hypochlorite [10]. PHB recovery using sodium hypochlorite treatment results in 95% recovery in recombinant E. coli. In the recovery step, the higher the concentration of the biomass, the better the recovery is. Yet, purity decreases as the concentration of biomass increases. PHB recovered using 20 % sodium hypochlorite yields 3.5% biomass concentration maximum and 83% purity (Figure 7). Due to the incompatibility between the biomass concentration and purity of PHB, sodium hypochlorite treatment does not satisfy the criteria required to make PHB production more cost efficient.
Another treatment for PHB recovery is SDS which has been tested to be easy to perform and costs much lower than sodium hypochlorite treatment. SDS can result in as high as 93% PHB when using biomass of 5% concentration (Figure 7). However, the percent recovery goes down as the biomass concentration goes up due to the higher amount of nucleic acid being released, which leads to the formation of suspension as well as an increase in viscosity.
Temperature and Time
The optimal treatment time for PHB recovery in SDS is 60 minutes at 30 ̊C [10].
Mixing substrates
One way to enhance the rate of PHB productivity is by using more than one carbon source. By using a mixture of acetate and glucose, instead of only acetate or only glucose, the efficiency of carbon conversion goes up to approximately 82% [9]. The addition of formate to methanol also shows positive feedback in productivity; it increases the efficiency from 22.3 to 36.7%. By having different sources of carbon, the pathway of PHB synthesis could avoid a possible dead-end route. Also, since the redox balance of the biosynthesis of PHB is not energetically neutral, mixing highly energetic and poorly energetic substrates can overcome this problem. For example, a mixture of glucose and acetate as substrates for PHB biosynthesis is effective in overcoming the problem mentioned previously because glucose is highly energetic and acetate is the opposite of glucose. Part of acetate must be used in the redox reaction to re-generate energy. In the presence of glucose, acetate does not have to undergo the degradation process. Instead, the making of PHB from glucose releases a sum of energy that can be utilized to assimilate acetate.
Other Roles and Drawbacks
Other roles of Alcaligenes eutrophus
Bioremediation Agent
Alcaligenes eutrophus have developed resistance to heavy metal ions through mutations and hence can grow in the presence those otherwise deadly ions [13]. In fact, this bacterium can drastically decrease the Cd2+ concentration in a culture from 200 ppm to 2 ppm as well as Zn2+ concentration from 600 ppm to 10 ppm. Therefore, A. eutrophus is particulary useful in treating industrial wastewater. Moreover, A. eutrophus can also bioremediate polluted soils. However, further research is necessary in order to gain a full understanding of how it can effectively function as soil bioremediation agent.
Nitrate Reductase
The H16 strain of Alcaligenes eutrophus can reduce the highly toxic nitrate [14]. Many bacteria use nitrate as their main source of nitrogen. For the nitrogen to be metabolized, it has to first be converted into nitrite and then to ammonia. The metabolism of nitrate can be anaerobic and aerobic. In many types of bacteria, such as Alcaligenes eutrophus H16, dinitrogen is the product of respiration, with nitrogen serving as final electron acceptor.
Some Drawbacks
Although biodegradable plastics might seem an environmentally friendly alternative to non-biodegradable plastics, there are some disadvantages. Degradation of oil-based biodegradable plastic may result in the release of carbon dioxide, a harmful greenhouse gas that participates in global warming. Degradation of starch-based biodegradable plastics may not have harmful byproducts such as CO 2 gas. However, they have the potential to contaminate soil and water [11].
The Oxo Biodegradable plastic bag is another type of biodegradable polymer which causes a number of concerns regarding metal wastes [12]. This type of plastic contains some metal salts, such as cobalt, iron or manganese, and degrades in the presence of sunlight and oxygen. Even though its degradation process takes a much shorter time than the petroleum-base plastic (which can take as long as hundreds of years), there are still possibilities that some of the metals will not be degraded. This is because the complete degradation of oxo biodegradable plastic is highly dependent on the micro organism in the environment. Also, since it needs oxygen and sunlight, the possibility of it starting to degrade drops to almost zero when it is buried in soil or in other waste. Oxo biodegradable plastic also will probably not degrade during the winter since its rate of catabolism goes down as temperature goes down and humidity goes up.
Conclusion
The usage of biodegradable polymer has significantly been going up as society becomes more concerned about the polluted environment due to petroleum-based plastic usage. However, in order for the biodegradable plastic to be more favorable than the "bad" plastic in the market, further researches focusing on how to make it more cost-efficient and yield better properties are necessary [15]. Some of the studies that have been done resulted in the discoveries of many ways of producing PHB through different sources of biomass. Some of them are cornstarch-based bioplastics, bioplastics from potatoes, sugarcane bioplastic, and mycelium-based bioplastic [16]. To date, using mycelium, a compound derived from mushrooms, is the most promising way of producing a stronger PHB with higher durability. Technically mycelium is not bioplastic, yet it has qualities and capacities that can serve as a substitute for plastics. Also, Gavin McIntyre and Eben Bayer discovered that using the self-assembling mycobond, another mushroom-derived material, in producing a packing alternative to conventional plastic reduces the production energy up to 98%.
Another research by Giannelis and colleagues led to the production of a biopolymer with a higer decomposition rate [17]. They found that nanoclays-modified PHB was stronger than the regular biodegradable plastics yet decomposes at a much higher rate. Additionally, they also found that adjusting the amount of nanoparticles added can control the rate of degradation of nancolays-modified PHB.
Overall, biopastics can be produced using different sources of biomass and by multiple techniques. However, the benefits of using bioplastics instead of oil-based plastics are similar: 1) less usage of landfill, 2) less pollution, 3) a decrease in waste accumulation, and 4) a reduced carbon footprint [16]. However, it is important to take note that not all bioplastics are degradable. Hence, if the purpose of switching from oil-based plastic usage to bioplastic usage is to sustain a better environment, it is crucial to make sure that the bioplastics that we use are decomposable.
References
[1] DOE Joint Genome Institution. US Department of Energy: Office of Science. Ralstonia metallidurans.
[8] Biotech Articles. 2010. Biodegradable Polymers— Production, Advantages, and Drawbacks.
[11] Wikipedia. Biodegradable Plastic: Advantages and Disadvantages.
[12] Pearce, F. 2009. The Guardian: Biodegradable plastic bags carry more ecological harm than good.
[15]FuturEnergia. 2009. Energy Is Our Future: Bioplastics and friends
[17]Science Daily. 2007. 'Nanohybrid' Plastic May Expand Use Of Biodegradable Plastic
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.