Amphibian Skin Microbiome
Introduction
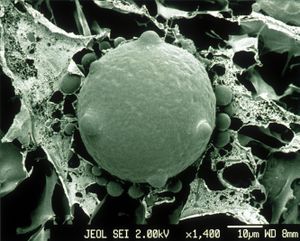
An abundance of bacteria and other microbes are known to thrive on the skin of amphibians. Many factors have been found to influence what kinds of microbes are found on the skin of amphibians, such as species type and environmental surroundings. For instance, within one species of frog, members of the same population have microbiomes that are more similar to one another than to individuals in different populations. This suggests that different amphibian species select for a specific microbiome, but are also colonized and influenced by environmental reservoirs (Mckenzie et al., 2012). In addition, the bacteria that grow on amphibian’s skin can either be harmful to the host or have a neutral or even beneficial effect. For instance, in a study conducted to analyze the potentially symbiotic role of bacteria growing on Rana Mucosa (Mountain Yellow-Legged Frog), it was found that the bacteria may contribute to the health of the innate immune system response of the frogs, in relation to the presence of the chytrid fungus, a harmful pathogen to amphibians (Woodhams et al, 2007). The amphibian skin microbiome is a recent area of research and numerous studies are being conducted to gain a further understanding of its dynamics.
Physical environment
The community structure of the amphibian skin microbiome is strongly associated with host species and is affected by a variety of factors.
Amphibian-Microbiome Relationship
A wide range of variables can influence microbiome-host interactions amongst amphibians. Factors ranging from genetics, life history, and behavior of the host to broader effects, such as habitat, are a few such factors (Ding&Schloss, 2014). More factors are still being discovered. For instance, recently it has been found that skin microbiomes can be associated and influenced by water conductivity, ratio of natural to managed land, and latitude (Katherine et al, 2016). Additionally, seasonality has also been correlated with the frog skin microbiome. In observing significant seasonal changes in skin microbial communities of adult Rana yavapalensi (Lowland Leopard Frog), Longo et al. (2015) found that skin microbial communities increase in diversity from summer to winter. This may be linked to temperature-influenced changes in the host immune function and bacterial growth. To further complicate the microbiome and host relationship, frog skin of different species and individuals may vary due to anti-microbial skin peptides present, secretions from mucosal membranes, and frequency of skin shedding (Van et al, 2015). All of these factors can influence the establishment and maintenance of resident microbial communities. Research is being conducted to gain a more complete understanding of the amphibian host-microbiome relationship, especially in hope of advancing conservation efforts and technology, such as probiotic therapy.
Microbial communities
Amphibian skin microbiome research has strived to identify key bacterial species and to understand their role in host-microbiome interactions. Through culture-based methods, 16S rRNA gene sequencing, and experimental trials, several researchers have identified some dominant microbial species present in the amphibian skin microbiome.
Dominant Microbes
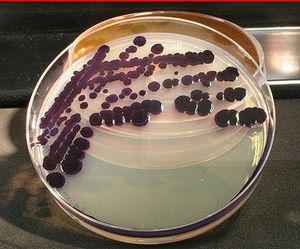
Janthinobacterium lividum
J. lividum is an aerobic, Gram-negative bacterium that resides in soil. It has a characteristic deep purple color that is attributable to a compound called violacein. Violacein is produced through the metabolism of glycerol as a carbon source and has been found to have anti-bacterial, anti-viral, and anti-fungal properties (Black, 2016). J. lividum's anti-fungal properties have been of particular interest since the bacterium has been found on the skin of amphibians, where it has been shown to prevent infection by pathogenic chytrid fungi (Becker et al. 2008).
Lysobacter gummosus
Lysobacter belongs to the family Xanthomonadaceae and L. gummosus is one of the 13 named species within the family. L. gummosus reside in various habitats of soils and water. Recently, L. gummosus has been found residing on the skin of redback salamanders and has been found to produce a chemical that can inhibit the growth of harmful pathogens (Bucker et al, 2008).
Other
Additional dominant bacterial phyla found on amphibian skin include Acidobacteria, Actinobacteria,Bacteriodetes ,Cyanobacteria ,Firmicutes , and Proteobacteria (Mckenzie, et al. 2012). More research is currently being done to further characterize microorganisms that reside on amphibian skin and the potential anti-fungal properties they may possess.
Association between skin microbiome and disease
The chytrid fungus, Batrachochytrium dendrobatidi, (Bd), has been a major focus of research, as the degree of susceptibility varies amongst species. Similar factors that determine the composition of the amphibian skin microbiome also affect the susceptibility of species to Bd ,e.g., climate and host density (Bielby et al. 2015). How the skin microbiome plays a part in mitigating susceptibility to disease has been demonstrated in various studies. The bacterium J. lividum has been shown to have anti-fungal properties at high concentrations (Becker et al. 2008). Other studies, through the use of lab trials, have demonstrated how such bacteria interact with host susceptibility. The effectiveness of the anti-fungal properties of Bd -inhibiting bacteria has been tested through the inoculation of susceptible species with antifungal bacteria, thus resulting in lower infection levels (Becker et al. 2009, Harris et al. 2009). Still other studies have demonstrated how the whole-microbiome approach is important. Beck and Harris (2010), showed that Plethodon cinerus (Red-Backed Salamander) was less resistant to Bd infection when cleared of indigenous microbiome bacteria prior to Bd exposure. This suggests that altering the microbiome can have major health consequences for the host. Knowledge obtained through such studies can help lead to field study applications and enhance knowledge ofBd disease patterns in wild amphibian populations. Studies involving Bd and skin associated microbes that impart resistance, show there is an interaction between the two, with both influencing the other's abundance and presence.
Current Research
Analysis and research on the amphibian microbiome is a recent area of study. However, it has important implications for conservation applications, though few studies have been conducted concerning this. Studying the microbiome can serve as a useful tool throughout the conservation process as it can be used to identify declining populations of amphibians (AmphibiaWeb, 2015). Many factors are linked to amphibian decline, but identifying strategies to combat Bd has been a major focus in amphibian conservation. Current research has shown that bioaugmentation with symbiotic bacteria is a reliable method for conserving Bd -susceptible species of amphibians. Bioaugmentation enhances host resistance to Bd through the addition of symbiotic bacteria. Although bioaugmentation has the potential to reduce Bd infection rates, most studies have only focused on a few species of bacteria. However, some researchers are taking on a whole-microbiome approach to conservation, with the initiation of "microbiomics" (AmphibiaWeb, 2015). As an example, Becker et al. (2014) highlighted that microbial community analyses can aid in prediction of susceptibility. It was found that individual microbiomes succumbing to Bd infection were distinct from those cleared of infection, and distinctions were even evident before a trial began (Becker et al. 2014). This showed that resident microbes were highly influential on the effects of disease outcome, thus suggesting the use of microbiome profiles as identification of populations that are at higher risk for disease. This in turn, can further enhance amphibian conservation strategies. Prevention of species decline and extinction is a sought-after goal amongst conservationists, but unfortunately, this is not always possible for every species. As a result, conservationists often turn to captive breeding programs. However, the captive environment is not the same as the wild environment. Thus, several studies have been conducted to test the effects of captivity on the amphibian microbiome. Three particular studies have has shown that there is a distinct difference in microbiome between captive and wild specimens. In the two studies, captive amphibians display microbiome diversity reduction (Antwis et al. 2014, London et al. 2014) while in the third, diversity increased (Becker et al, 2014). These current studies demonstrate that an incorporation of amphibian microbiomes with conservation research may enhance the success of captive breeding programs.
References
AmphibiaWeb: Information on amphibian biology and conservation. [web application]. 2015. Berkeley, California: AmphibiaWeb. Available: http://amphibiaweb.org/. Accessed: 26 Mar, 2016 ).
Antwis, R. E., R. L. Haworth, D. J. P. Engelmoer, V. Ogilvy, A. L. Fidgett, and R. F. Preziosi. 2014. Ex situ diet influences the bacterial community associated with the skin of red-eyed tree frogs (Agalychnis callidryas). PloS one 9:e85563.
Becker M & R Harris. 2010. Cutaneous bacteria of the redback salamander prevent morbidity associated with a lethal disease. PLoS One 5 (6): e10957.
Becker, M. H., C. L. Richards-Zawacki, B. Gratwicke, and L. K. Belden. 2014. The effect of captivity on the cutaneous bacterial community of the critically endangered Panamanian golden frog (Atelopus zeteki). Biological Conservation 176:199–206.
Black, R. E. 2016. Investigating genetic differences between strains of Janthinobacterium lividum on salamanders and in their environment. University of Tennessee Honors Thesis Projects. http://trace.tennessee.edu/utk_chanhonoproj/1871.
Brucker RM, Baylor CM, Walters RL, Lauer A, Harris RN, Minbiole KPC. 2008. The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. Journal of Chemical Ecology 34(1):39-43.
Bielby J, MC Fisher, FC Clare, GM Rosa, TWJ Garner. 2015. Host species vary in infection probability, sub-lethal effects, and costs of immune response when exposed to an amphibian parasite. Nature Scientific Reports 5: e10828.
Ding T & PD Schloss. 2014. Dynamics and associations of microbial community types across the human body. Nature 509: 357-360.
Longo, A. V., A. E. Savage, I. Hewson, and K. R. Zamudio. 2015. Seasonal and ontogenetic variation of skin microbial communities and relationships to natural disease dynamics in declining amphibians. Royal Society Open Science 2:140377.
Loudon, A. H., J. A. Holland, T. P. Umile, E. A. Burzynski, K. P. C. Minbiole, R. N. Harris, and A. Loudon. 2014. Interactions between amphibians ’ symbiotic bacteria cause the production of emergent anti-fungal metabolites. Frontiers in Microbiology 5 (441): 1-8.
McKenzie, V. J., R. M. Bowers, N. Fierer, R. Knight, and C. L. Lauber. 2012. Co-habiting amphibian species harbor unique skin bacterial communities in wild populations. The ISME Journal 6: 588–96.
Van Rooij, P., Martel, A., Haesebrouck, F., & Pasmans, F. 2015. Amphibian chytridiomycosis: a review with focus on fungus-host interactions. Veterinary Research, 46, 137. http://doi.org/10.1186/s13567-015-0266-0
Woodhams, D. C., Vredenburg, V. T., Simon, M. A., Billheimer, D., Shakhtour, B., Shyr, Y., & Harris, R. N. 2007. Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biological conservation, 138(3),390-398.
Edited by Autumn Fish, a student of Mary Beth Leigh at the University of Alaska Fairbanks Template adapted from one used by Angela Kent at the University of Illinois at Urbana-Champaign.