Avian Malaria in Hawaiian Birds
Jess Kotnour
Plasmodium
While malaria is most commonly thought of in terms of the few species that infect humans, there are several hundreds of haemosporidian parasites that infect a variety of vertebrate hosts [1]
These parasites are commonly split into five genera: Plasmodium, Hepatocystis, Haemoproteus, Parahaemoproteus, and Leucocoytozoon [2]. Of these genera, species within the Leucocytozoon, Parahaemorptoeus, and Plasmodium genera have been found to infect avian species [3]. Of these, Plasmodium relictum has been devastating the native birds on the island of Hawaii [4]. P. relictum and the native birds of Hawaii did not co-evolve, which is one of the main reasons why the native populations are so negatively impacted by this parasite. While the exact date of introduction in unknown, before the 1800s, Hawaii was not home to any mosquitos [5]. Non-native birds were first introduced to the island in the 1800s. In the late 1800s, avian pox is reported for the first time on Hawaii by naturalists who noticed tumor-like growths, a sign of avian pox, on dead birds in the forests [6]. Their observation has been verified with modern science; avian poxvirus has been found using PCR amplification on museum specimens collected during that time frame [7]. While avian pox and avian malaria may have arrived at the same time, P. relictum was first observed on Hawaii in the 1930s on blood smears from a red-billed leiothrix [8]. The spread of both avian malaria and avian pox were thought to be compounded by the introduction of non-native passerines by bird clubs who would bring non-native birds, and their diseases, from all corners of the world to Hawaii [9].
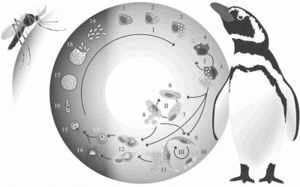
Life Cycle of Plasmodium relictum
Plasmodium relictum follows the common haemosporidian life cycle. P. relictum initially undergoes a period of asexual reprodcution within mosquito host tissues [11]. This initial cycle is followed by subsequent cycles of asexual reproduction within both host tissues and red blood cells, leading to gametocytes within host red blood cells [12]. These gametocytes will remain in the midgut, becoming gametes until forming fertile zygotes [13]. These zygotes will eventually form into oocysts. Inside the oocysts, asexual reproduction will occur to form sporozoties [14]. As they rupture from oocysts, these sporozoties will move into the salivary glands of the mosquito, where they can be transmitted intravenously, intramuscularly, or intraperitoneally to birds [15]. These gametocytes will remain in the midgut, becoming gametes until forming fertile zygotes [16].
Once inside the avian host, the life cycle continues. The sporozoties will invade the avian host’s reticuloendothelial (macrophages and monocytes) cells where they will develop into mernots [17]. Through asexual reproduction, mernots will reproduce into merozites while still inside the host’s reticuloendothelial cells [18]. When the cell inevitably ruptures, the merozites will be released and will spread throughout the host using the blood stream [19]. P. relictum continues its development in reticuloendothelial and host tissues [20]. They most commonly develop in the liver, spleen, and kidneys of the host [21]. Once inside either the host’s cells or tissue, the merozites will further develop into either erythrocytic mernots or gametocytes [22]. If the merozite develops into a gametocyte, the development is complete, and it will remain in the host’s red blood cells until a mosquito vector bites the host [23]. If the host is bitten by a mosquito, the gametocyte will repeat the life cycle in the mosquito.
P. relictum often infects birds most often during the breeding months [24]. Breeding season is often during the warmer months of the year, allowing for the development mosquitos and P. relictum [25]. These mosquitos are able to take advantage of the breeding season, as birds are often investing more resources into raising their young than their immune systems [26]. The weakened immune system makes infection by P. relictum much easier [27].
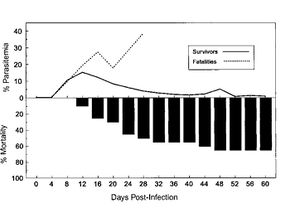
Common impact of P. relictum on birds
While symptoms of P. relictum infection may vary from individual from individual, the infection normally follows a predictable course. After the initial infection, the bird will undergo an acute period lasting 6-12 days [29]. The intensity of the infection will then increase. This intensity can be effected by a variety of factors, from the host's immune system to the season [30]. At peak intensity, birds will commonly be anorexic [31]. Their feathers will be in poor condition, and their body weight will decrease due to the anorexia and other factors [32]. They will often be anemic and have a low hematocrit, as seen in their thin, watery blood [33]. Both the liver and spleen will be enlarged and discolored, due to the large parasite load often found in these organs [34]. Avian malaria is normally diagnosed through blood samples [35]. A small blood sample will be taken in order to make a blood smear on a slide [36]. Once blood smears are fixed, the number of infected red blood cells are counted using microscopy [37]. In addition to the common method of looking at infected erythrocytes using microscopy, newer diagnostic techniques are starting to be used [38]. One of these techniques is the use of PCR [39]. DNA will be extracted from blood samples, and the mitochondrial cytochrome b gene from the parasite will be amplified [40]. If this gene is present, it can be concluded that the bird does have avian malaria. In some studies, samples that test positive for the parasite cytochrome b gene will be confirmed through a second PCR that will amplify the ribosomal DNA of P. relictum [41].
Current vector and its habitat
While the initial Hawaiian vector of P. relictum is unknown, Culex quinquefasciatus , the southern house mosquito, is the current vector. C. quinquefasciatus needs standing water in order to lay larva and has found this throughout Hawaii, again due to human impacts. On the Mauna Loa Volcano, larvae were found at altitudes up to 1500 m during all parts of the year [43]. Larvae were most commonly found in tree cavities that were made by feral pigs [44]. Feral pigs often eat the inner corn of tree ferns, creating cavities that will fill with water, providing C. quinquefasciatus larval habitat [45]). In addition to this natural habitat, larvae were also found in large water troughs [46]. In the drier parts of Hawaii, such as the mesic-dry forests, rock holes in begs of stream drainages provided most of the larvae habitat [47]. In these drier parts, anthropogenic sources of standing water provide most of the larval habitat [48].
Like all living organisms, C. quinquefasciatus and P. relictum are impacted by their environment. The development rates of C. quinquefasciatus and P. relictum both depend heavily on rainfall and temperature. While rainfall across Hawaii is fairly consistent, during El Nino there is significant lower rainfall [49]. This decline of rainfall leads to less available larval habitat, and subsequently fewer mosquitos available to transmit malaria [50]. Since Hawaii is fairly near the equator, the temperature does not vary that much seasonally. The main temperature variation comes from the elevation changes provided from Hawaii's mountains [51]. P. relictum needs a minimum temperature of 13 °C for development, but develops most rapidly at temperatures above 28 °C (LaPointe 2013). Because of this, at lower elevations, mosquitos and P. relictum are able to thrive. At elevations greater than 1,700 meters, there is very little larval habitat. The lower temperatures at this altitude makes this unsuitable habitat for mosquito and P. relictum development. Because of this, there is limited spread of avian malaria at these elevations.
As global temperatures rise and lead to changing precipitation patterns, larval habitat may be positively affected for C. quinquefasciatus (Atkinson et al 2014). On the Alaka’I Plateau, temperatures are rising at a trend of .119 °C per decade [52]. In addition to higher temperatures, the plateau has become drier over the years of the study, although this is not leading to a decrease in larval habitat [53]. The decrease in precipitation, however, may actually lead to an increase in mosquitos. Precipitation leads to high streamflow events, which would normally disrupt the breeding of C. quinquefasciatus at their stream margin breeding sites on the plateau. As precipitation decreases, the high streamflow events will also decrease, allowing C. quinquefasciatus to remain longer to breed. This does and will continue to increase the mosquito population and the spread of avian malaria. At three locations across the Plateau, the prevalence of malaria doubled from 8.6% to 19.6% [54]. Other higher elevation locations around Hawaii are also seeing an increase of avian malaria. The prevalence of malaria at 1,800m at Hakalau Forest National Wildlife refuge increased from 2.1% to 5.4% [55]. These changes are also thought to be due to the warming of the climate [56].
Current State of Native Hawaii Birds
Avian malaria is of particular interest on the islands that make up Hawaii due to the decline of native bird populations over the past 200 years. In the forests of Kona, there are almost no native birds present; nonnative species have completely taken over this habitat [57]. The decline of native birds was first shown through the work of Helen James and Storrs Olson, who calculated that at least 50% of Hawaiian avifauna have gone extinct [58]. While there was 100 endemic species known to have lived on the island, only 35 are still extant [59].
Honeycreepers are one group of native Hawaiian birds that have seen a great decline over the past few hundred years. Honeycreepers are a varied group of passerines that are found mainly in forests [60]. While there were once 54 species of honeycreepers, their numbers have been declining as well [61]. 14 species went extinct prior to the arrival of the westerners, as shown through the fossil record, yet 14 more species have been lost since the arrival of westerners [62]. Of the 26 species of honeycreepers that remain, 18 of these species are considered endangered [63]. One of the biggest threats to Honeycreepers is the elevational rise in habitat of C. quinquefasciatus and the spread of avian malaria [64]. The upward movement of C. quinquefasciatus have made Molokai, Lanai, and Oahu basically inhabitable for honeycreepers [65].
Why the devastation?
While avian malaria normally does not cause this amount of devastation in avifauna, the majority of continental avifauna have coevolved with mosquitos and their pathogens [66]. Since mosquitos and avian malaria were not introduced to Hawaii until fairly recently, native avifauna are just now learning how to deal with this new pathogen.
While the high mortality rate of avian malaria on Hawaiian birds can be deduced from the extinction of the honeycreepers, similar results have been replicated in the lab. Atkinson et al conducted an experiment where Amakihi were infected by bite from a sole P. relictum infected mosquito. All 20 of the birds infected developed a P. relictum infection within 8 days of the initial exposure [67]. Of the 20 that were infected, 13 died. Nine died from acute infections while the other four died from complications. While seven birds did survive, they lost a significant amount of body weight and were acutely ill, which may have led to their death in natural conditions [68]. Despite this high mortality rate, when a similar experiment was done with the I’iwi, mortality rates were around 90% [69].
Non-native Pathogens
One explanation for the terrible impact that P. relictum has had on native Hawaiian populations is the novel weapons hypothesis [70]. When a new species, such as the birds that the bird clubs brought to Hawaii, is introduced to an environment, they bring with them new pathogens that the native population has never encountered before. These novel pathogens negatively impact the native population more than the invader, since the native population has never encountered the novel pathogen before. This gives the invading species an advantage, often driving the native species to extinction. This phenomenon has been documented in several species throughout history. In recent times grey squirrels were introduced to England [71]. These squirrels brought with them a poxvirus that was novel to the native red squirrel population [72]. This poxvirus killed off the majority of red squirrels in England, allowing the grey squirrels to essentially take over [73].
Currently, a novel pathogen is killing the native amphibians across North America [74]. Batrachochytrium dendrobatidis , a fungus, was introduced to North American amphibians by an invasive bullfrog species, Rana catesbeiana [75]. While this fungus normally is not deadly to R. catesbeiana , it has decimated native populations of amphibians [76]. The plight of the native Hawaiian birds is a classic example of the novel weapons hypothesis.
Modeling the Future Spread of Avian Malaria
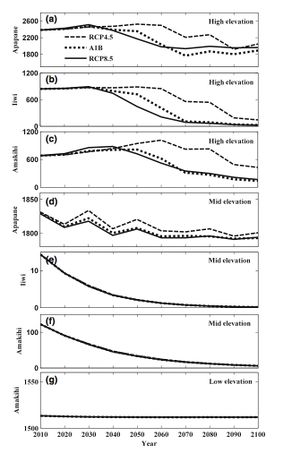
Although avian malaria has already devastated native Hawaiian species, its future impacts are still unknown. In order to predict the future effects of avian malaria, modeling can be used. As global temperature increase, the effects of climate change need to be taken into account while modeling is done.
Using three different models, the effects of varying amounts of temperature and rain changes were explored [78]. Under the RCP4.5 model, the effects of a warmer and dryer climate were modeled [79]. RCP8.5 was used to explore hot and dry conditions, and A1B modeled a warm and wet Hawaii. RCP8.5 and A1B predicted the largest devastation of honeycreepers due to avian malaria [80].
Using these models, temperature increases for low, medium, and high elevations were predicted. In all three models, high elevations saw the greatest increase in temperature over the next 100 years [81]. RCP8.5 predicted a 4.3° C temperature increase at high elevations, and only a 3.9 ° C increase at low elevations [82]. Under this same model, rainfall was expected to decrease in both the west and dry seasons at all elevations. Under A1B, rainfall increased in both seasons at all elevations, except during the wet season at low elevations [83]. This high rainfall model was predicted to increase relative mosquito abundance across all elevations [84]. Although birds have sought refuge from malaria at high elevations, this malaria-free refuge would remain until 2040, when mosquitos are expected to spread to high elevations [85].
With the spread of mosquitos, the annual malaria transmission is expected to increase at middle to high elevations [86]. While the current transmission rate of malaria at middle elevations is .85, it will reach maximum transmission rates by the middle of the century [87]. The rate of transmission at high elevations is not predicted to reach the maximum transmission rate in any of the three models, but is expected to increase to between .3 to .8, depending on the model [88].
Under all three of the models, populations of three species of Hawaiian honeycreepers are expected to decrease [89]. The I’iwi is expected to decline at high elevations by 70-90% by 2100 [90]. Since the Apapane is less susceptible to malaria than the I’iwi, it is predicted to decline by only 10-20% at high elevations [91]. The only native bird predicted to persist at low-elevation forests was the Amakihi [92].
While these predictions may seem dire for the Hawaiian honeycreepers, increased infection and major population declines were not predicted until nearly 2040 [93]. This buffer may provide time for conservation strategies to be implemented.
The Amakihi
As Atkinson showed, the Amakihi has much lower mortality rates of avian malaria than the I’iwi does. One of the proposed mechanisms behind the differences in mortality rates in the major histocompatibility complex, Mhc. The Mhc a key part of the adaptive immune system and is responsible for binding fragments from pathogens and displaying these fragments on the cell surface [94]. Genetic variation of the Mhc class II chain peptide-binding region was explored in both the Amakihi and the I'iwi [95]. While it was proposed that the Amakihi would have higher variety of Mhc's than the I'iwi, there was no difference in the Mhc sequence variability at the 13 PBC sites [96]. However, there was lower diversity in the 32 non-PBC sites in the I'iwi [97]. While these differences still need to be explored further, they provide an initial pathway for understanding the way that the Honeycreepers can survive this avian malaria epidemic.
The Amakihi are not only unique in the Mhc diversity, but in other parts of their genome as well. Amakihi was highly differentiated in both their mitochondrial DNA and nuclear markers, showing that this "resistant" low-elevation population was rather unique on the island (Foster et al 2007). This diversity points to in situ population growth of these resistant birds. This population of resistant birds was most likely made from remnants of low-elevation populations, rather than a migration of high-elevation population [98].
Why would this one species show this resistance? One theory has to do with their life history. Both the I’iwi and Apane have larger ranges of movements for their food sources, whereas the Amakihi tends to remain rather sedentary [99]. By remaining in one area, the Amakihi is under stronger selective pressures than the I’iwi and Apane [100]. This pressures may have selected for the Amakihi’s resistance to avian malaria.
Conservation Directions
Vaccinations and medications
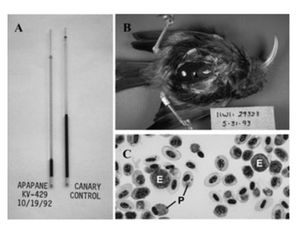
One avenue to be explored is the possibility of vaccinating the remaining population of honeycreepers against malaria. While vaccines against P. relictum have been have been developed, they have not been tested in honeycreepers [101]. These vaccines did provide protection to penguins and canaries, but the protection was not long lasting [102]. Given the resources it would take to vaccinate enough individuals in this population to have a resistant population, vaccination seems like an unlikely route to conserving the native birds.
On a similar vein, anti-malarial drugs have been successful in treating malaria in other species of birds [103]. The disadvantages to anti-malarial drugs are similar to those of vaccinating. Even if the drugs are successful in treating the infection, it would be extremely difficult to treat all infected individuals. Anti-malarial drugs also could lead to a resistant population of P. relictum , similar to the antibiotic-resistant infections that are common in hospitals today.
Limiting C. quinquefasciatus habitat
If the population of C. quinquefasciatus could be reduced, then the infection rates of P. relictum would decrease as well. In order to reduce the C. quinquefasciatus population, larval habitat would need to be greatly minimized. Since feral pigs are responsible for creating tree holes that are prime larval habitat, controlling the feral pig population would significantly help the native birds [104]. While an elimination of feral pigs would be ideal from a conservation standpoint, feral pigs are a common game species [105]. Their elimination would most likely lead to much controversy. While natural sources of C. quinquefasciatus habitat may not be able to be entirely removed, anthropogenic larval habitats, such as water troughs, could also be removed to limit larval development habitat [106].
Larval habitat needs to be almost completely removed, however, in order for there to be significant benefits on the native population [107]. If only some larval habitat is removed, yet the majority remains, we will likely continue down the path towards extinction of the native avifauna.
References
- ↑ Martinsen and Perkins 2013. The Diversity of Plasmodium and other Haemosporidians: The Intersections of Taxonomy, Phylogenetics, and Genomics. In: Carlton, J., Perkins, S., Deitsch, editors. Malaria Parasites: Comparative Genomics, Evolution and Molecular Biology. Norfolk: Caister Academic Press. p 1- 15.
- ↑ Martinsen and Perkins 2013. The Diversity of Plasmodium and other Haemosporidians: The Intersections of Taxonomy, Phylogenetics, and Genomics. In: Carlton, J., Perkins, S., Deitsch, editors. Malaria Parasites: Comparative Genomics, Evolution and Molecular Biology. Norfolk: Caister Academic Press. p 1- 15.
- ↑ Martinsen and Perkins 2013. The Diversity of Plasmodium and other Haemosporidians: The Intersections of Taxonomy, Phylogenetics, and Genomics. In: Carlton, J., Perkins, S., Deitsch, editors. Malaria Parasites: Comparative Genomics, Evolution and Molecular Biology. Norfolk: Caister Academic Press. p 1- 15.
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ Atkinson, C., LaPointe, D. 2009. Introduced Avian Diseases, Climate Change, and the Future of Hawaiian Honeycreepers. Journal of Avian Medicine and Surgery. 23 (1): 53-63.
- ↑ Henshaw, H. 1902. Complete list of the Birds of the Hawaiian Possessions with Notes on their Habits. Honolulu: Thos. G. Thrum.
- ↑ Atkinson, C., LaPointe, D. 2009. Introduced Avian Diseases, Climate Change, and the Future of Hawaiian Honeycreepers. Journal of Avian Medicine and Surgery. 23 (1): 53-63.
- ↑ Fisher, H., Baldwin, P. 1947. Notes on the Red-billed Leiothrix in Hawaii. Pacific Science. 45-51.
- ↑ Atkinson, C., LaPointe, D. 2009. Introduced Avian Diseases, Climate Change, and the Future of Hawaiian Honeycreepers. Journal of Avian Medicine and Surgery. 23 (1): 53-63.
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ Atkinson, C., van Riper, III, C. 1999. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loyle J. and Zuk M., editors. Bird-Parasite Interactions. Oxford University Press. p 19-48
- ↑ Atkinson, C., van Riper, III, C. 1999. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loyle J. and Zuk M., editors. Bird-Parasite Interactions. Oxford University Press. p 19-48
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ Grilo, M., Vanstreels, R., Wallace, R., García-Párraga, D., Braga, É., Chitty, J., Catão-Dias, J., Madeira de Carvalho, L. 2016. Malaria in penguins—current perceptions. Avian Pathology. 45(4): 393-407
- ↑ Grilo, M., Vanstreels, R., Wallace, R., García-Párraga, D., Braga, É., Chitty, J., Catão-Dias, J., Madeira de Carvalho, L. 2016. Malaria in penguins—current perceptions. Avian Pathology. 45(4): 393-407
- ↑ Grilo, M., Vanstreels, R., Wallace, R., García-Párraga, D., Braga, É., Chitty, J., Catão-Dias, J., Madeira de Carvalho, L. 2016. Malaria in penguins—current perceptions. Avian Pathology. 45(4): 393-407
- ↑ Grilo, M., Vanstreels, R., Wallace, R., García-Párraga, D., Braga, É., Chitty, J., Catão-Dias, J., Madeira de Carvalho, L. 2016. Malaria in penguins—current perceptions. Avian Pathology. 45(4): 393-407
- ↑ Grilo, M., Vanstreels, R., Wallace, R., García-Párraga, D., Braga, É., Chitty, J., Catão-Dias, J., Madeira de Carvalho, L. 2016. Malaria in penguins—current perceptions. Avian Pathology. 45(4): 393-407
- ↑ Grilo, M., Vanstreels, R., Wallace, R., García-Párraga, D., Braga, É., Chitty, J., Catão-Dias, J., Madeira de Carvalho, L. 2016. Malaria in penguins—current perceptions. Avian Pathology. 45(4): 393-407
- ↑ Grilo, M., Vanstreels, R., Wallace, R., García-Párraga, D., Braga, É., Chitty, J., Catão-Dias, J., Madeira de Carvalho, L. 2016. Malaria in penguins—current perceptions. Avian Pathology. 45(4): 393-407
- ↑ Atkinson, C., van Riper, III, C. 1999. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loyle J. and Zuk M., editors. Bird-Parasite Interactions. Oxford University Press. p 19-48
- ↑ Atkinson, C., van Riper, III, C. 1999. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loyle J. and Zuk M., editors. Bird-Parasite Interactions. Oxford University Press. p 19-48
- ↑ Atkinson, C., van Riper, III, C. 1999. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loyle J. and Zuk M., editors. Bird-Parasite Interactions. Oxford University Press. p 19-48
- ↑ Atkinson, C., van Riper, III, C. 1999. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loyle J. and Zuk M., editors. Bird-Parasite Interactions. Oxford University Press. p 19-48
- ↑ [https://www.tandfonline.com/doi/full/10.1080/03079457.2016.1149145 Grilo et al.2016. Malaria in penguins—current perceptions. Avian Pathology. 45(4): 393-407. ]
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ Atkinson, C., Dusek, R., Woods, K., Iko, W. 2000. Pathogenicity of avian malaria in experimentally infected Hawaii Amakihi. Journal of Wildlife Diseases. 36(2): 197-204.
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ Atkinson, C., Dusek, R., Woods, K., Iko, W. 2000. Pathogenicity of avian malaria in experimentally infected Hawaii Amakihi. Journal of Wildlife Diseases. 36(2): 197-204.
- ↑ Atkinson, C., Dusek, R., Woods, K., Iko, W. 2000. Pathogenicity of avian malaria in experimentally infected Hawaii Amakihi. Journal of Wildlife Diseases. 36(2): 197-204.
- ↑ Atkinson, C., Dusek, R., Woods, K., Iko, W. 2000. Pathogenicity of avian malaria in experimentally infected Hawaii Amakihi. Journal of Wildlife Diseases. 36(2): 197-204.
- ↑ Atkinson, C., Dusek, R., Woods, K., Iko, W. 2000. Pathogenicity of avian malaria in experimentally infected Hawaii Amakihi. Journal of Wildlife Diseases. 36(2): 197-204.
- ↑ Atkinson, C., Urzurrum, R., LaPointe, D., Camp, R., Crampton, L., Foster, J., Giambelluca, T. 2014. Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands—an ongoing conservation crisis on the island of Kaua’i. Global Change Biology. 20: 2426-2436.
- ↑ Atkinson, C., Urzurrum, R., LaPointe, D., Camp, R., Crampton, L., Foster, J., Giambelluca, T. 2014. Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands—an ongoing conservation crisis on the island of Kaua’i. Global Change Biology. 20: 2426-2436.
- ↑ Atkinson, C., Urzurrum, R., LaPointe, D., Camp, R., Crampton, L., Foster, J., Giambelluca, T. 2014. Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands—an ongoing conservation crisis on the island of Kaua’i. Global Change Biology. 20: 2426-2436.
- ↑ Atkinson, C., Urzurrum, R., LaPointe, D., Camp, R., Crampton, L., Foster, J., Giambelluca, T. 2014. Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands—an ongoing conservation crisis on the island of Kaua’i. Global Change Biology. 20: 2426-2436.
- ↑ Atkinson et al. 2000. Pathogenicity of avian malaria in experimentally infected Hawaii Amakihi. Journal of Wildlife Diseases. 36(2): 197-204
- ↑ Goff, M., van Ripper III, C. 1980. Distribution of Mosquitoes (Diptera: Culicidae) on the East Flank of Mauna Loa Volcano, Hawaii. Pacific Insects. 22 (1-2): 178-188.
- ↑ Goff, M., van Ripper III, C. 1980. Distribution of Mosquitoes (Diptera: Culicidae) on the East Flank of Mauna Loa Volcano, Hawaii. Pacific Insects. 22 (1-2): 178-188.
- ↑ [https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1948-7134.2009.00028.x Nogueria-Filho, S, Nogueria, S., Fragoso, J. 2009. Ecological impacts of feral pigs in the Hawaiian Islands. Biodiversity Conservation. 18: 3677-3683.]
- ↑ Goff, M., van Ripper III, C. 1980. Distribution of Mosquitoes (Diptera: Culicidae) on the East Flank of Mauna Loa Volcano, Hawaii. Pacific Insects. 22 (1-2): 178-188.
- ↑ Reiter, M., and LaPointe, D. 2009. Larval habitat for the avian malaria vector Culex quinquefasciatus (Diptera: Culicidae) in altered mid-elevation mesic-dry forests in Hawai’i. Journal of Vector Ecology. 34: 208-216.
- ↑ Reiter, M., and LaPointe, D. 2009. Larval habitat for the avian malaria vector Culex quinquefasciatus (Diptera: Culicidae) in altered mid-elevation mesic-dry forests in Hawai’i. Journal of Vector Ecology. 34: 208-216.
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ Atkinson, C., Urzurrum, R., LaPointe, D., Camp, R., Crampton, L., Foster, J., Giambelluca, T. 2014. Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands—an ongoing conservation crisis on the island of Kaua’i. Global Change Biology. 20: 2426-2436.
- ↑ Atkinson, C., Urzurrum, R., LaPointe, D., Camp, R., Crampton, L., Foster, J., Giambelluca, T. 2014. Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands—an ongoing conservation crisis on the island of Kaua’i. Global Change Biology. 20: 2426-2436.
- ↑ Atkinson, C., Urzurrum, R., LaPointe, D., Camp, R., Crampton, L., Foster, J., Giambelluca, T. 2014. Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands—an ongoing conservation crisis on the island of Kaua’i. Global Change Biology. 20: 2426-2436.
- ↑ Atkinson, C., Urzurrum, R., LaPointe, D., Camp, R., Crampton, L., Foster, J., Giambelluca, T. 2014. Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands—an ongoing conservation crisis on the island of Kaua’i. Global Change Biology. 20: 2426-2436.
- ↑ Atkinson, C., Urzurrum, R., LaPointe, D., Camp, R., Crampton, L., Foster, J., Giambelluca, T. 2014. Changing climate and the altitudinal range of avian malaria in the Hawaiian Islands—an ongoing conservation crisis on the island of Kaua’i. Global Change Biology. 20: 2426-2436.
- ↑ Scott, M., Conant, S., van Ripper III, C. 2001. Introduction. Studies in Avian Biology. 22: 1-12.
- ↑ Scott, M., Conant, S., van Ripper III, C. 2001. Introduction. Studies in Avian Biology. 22: 1-12.
- ↑ Scott, M., Conant, S., van Ripper III, C. 2001. Introduction. Studies in Avian Biology. 22: 1-12.
- ↑ Pratt, D. & Bonan, A. (2018). Hawaiian Honeycreepers (Drepanididae). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive.
- ↑ Jarvi, S. Tarr, C., McIntosh, C., Atkinson, C, Fleischer, R. 2014. Natural selection of the major histocompatibility complex (Mhc) in Hawaiian honeycreepers (Drepanidinae). Molecular Ecology. 13: 2157-2168.
- ↑ Jarvi, S. Tarr, C., McIntosh, C., Atkinson, C, Fleischer, R. 2014. Natural selection of the major histocompatibility complex (Mhc) in Hawaiian honeycreepers (Drepanidinae). Molecular Ecology. 13: 2157-2168.
- ↑ Jarvi, S. Tarr, C., McIntosh, C., Atkinson, C, Fleischer, R. 2014. Natural selection of the major histocompatibility complex (Mhc) in Hawaiian honeycreepers (Drepanidinae). Molecular Ecology. 13: 2157-2168.
- ↑ Pratt, D. & Bonan, A. (2018). Hawaiian Honeycreepers (Drepanididae). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive.
- ↑ Pratt, D. & Bonan, A. (2018). Hawaiian Honeycreepers (Drepanididae). In: del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A. & de Juana, E. (eds.). Handbook of the Birds of the World Alive.
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ Atkinson, C., Dusek, R., Woods, K., Iko, W. 2000. Pathogenicity of avian malaria in experimentally infected Hawaii Amakihi. Journal of Wildlife Diseases. 36(2): 197-204.
- ↑ Atkinson, C., Dusek, R., Woods, K., Iko, W. 2000. Pathogenicity of avian malaria in experimentally infected Hawaii Amakihi. Journal of Wildlife Diseases. 36(2): 197-204.
- ↑ Atkinson, C., Dusek, R., Woods, K., Iko, W. 2000. Pathogenicity of avian malaria in experimentally infected Hawaii Amakihi. Journal of Wildlife Diseases. 36(2): 197-204.
- ↑ [https://www.springer.com/us/book/9783319451190 Morand, S. 2017. Infections and Diseaess in Wildlife by Non-native Organisms. In: Vilá, M., Hulme, P, editors. Impact of Biological Invasions on Ecosystem Services. Switzerland: Springer. p. 177-190. ]
- ↑ [https://www.springer.com/us/book/9783319451190 Morand, S. 2017. Infections and Diseaess in Wildlife by Non-native Organisms. In: Vilá, M., Hulme, P, editors. Impact of Biological Invasions on Ecosystem Services. Switzerland: Springer. p. 177-190. ]
- ↑ [https://www.springer.com/us/book/9783319451190 Morand, S. 2017. Infections and Diseaess in Wildlife by Non-native Organisms. In: Vilá, M., Hulme, P, editors. Impact of Biological Invasions on Ecosystem Services. Switzerland: Springer. p. 177-190. ]
- ↑ [https://www.springer.com/us/book/9783319451190 Morand, S. 2017. Infections and Diseaess in Wildlife by Non-native Organisms. In: Vilá, M., Hulme, P, editors. Impact of Biological Invasions on Ecosystem Services. Switzerland: Springer. p. 177-190. ]
- ↑ [https://www.springer.com/us/book/9783319451190 Morand, S. 2017. Infections and Diseaess in Wildlife by Non-native Organisms. In: Vilá, M., Hulme, P, editors. Impact of Biological Invasions on Ecosystem Services. Switzerland: Springer. p. 177-190. ]
- ↑ [https://www.springer.com/us/book/9783319451190 Morand, S. 2017. Infections and Diseaess in Wildlife by Non-native Organisms. In: Vilá, M., Hulme, P, editors. Impact of Biological Invasions on Ecosystem Services. Switzerland: Springer. p. 177-190. ]
- ↑ [https://www.springer.com/us/book/9783319451190 Morand, S. 2017. Infections and Diseaess in Wildlife by Non-native Organisms. In: Vilá, M., Hulme, P, editors. Impact of Biological Invasions on Ecosystem Services. Switzerland: Springer. p. 177-190. ]
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Liao et al.: Will a warmer and wetter future cause extinction of native Hawaiian forest birds?. Global Change Biology. 2015. 21:4342-4352-226
- ↑ Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The major histocompatibility complex and its functions.
- ↑ Jarvi, S. Tarr, C., McIntosh, C., Atkinson, C, Fleischer, R. 2014. Natural selection of the major histocompatibility complex (Mhc) in Hawaiian honeycreepers (Drepanidinae). Molecular Ecology. 13: 2157-2168.
- ↑ Jarvi, S. Tarr, C., McIntosh, C., Atkinson, C, Fleischer, R. 2014. Natural selection of the major histocompatibility complex (Mhc) in Hawaiian honeycreepers (Drepanidinae). Molecular Ecology. 13: 2157-2168.
- ↑ Jarvi, S. Tarr, C., McIntosh, C., Atkinson, C, Fleischer, R. 2014. Natural selection of the major histocompatibility complex (Mhc) in Hawaiian honeycreepers (Drepanidinae). Molecular Ecology. 13: 2157-2168.
- ↑ Foster, J., Woodworth, B., Eggert, L., Hart, P., Palmer, D., Duffy, D., Fleischer, R. 2007. Genetic structure and evolved malaria resistance in Hawaiian honeycreepers. Molecular Ecology. 16: 4738-4746
- ↑ Foster, J., Woodworth, B., Eggert, L., Hart, P., Palmer, D., Duffy, D., Fleischer, R. 2007. Genetic structure and evolved malaria resistance in Hawaiian honeycreepers. Molecular Ecology. 16: 4738-4746
- ↑ Foster, J., Woodworth, B., Eggert, L., Hart, P., Palmer, D., Duffy, D., Fleischer, R. 2007. Genetic structure and evolved malaria resistance in Hawaiian honeycreepers. Molecular Ecology. 16: 4738-4746
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.
