Bacterial Ice Nucleation
Overview
Bacterial Ice Nucleation is the biogenic catalysis of ice formation by bacteria and is a rare example of a living organism catalyzing phase transition. Notably, ice nucleation active bacteria are capable of seeding ice formation at much warmer temperatures than most non-living particles.[1]
Distribution of Trait
Species with ice nucleation activity (INA) are frequently found in association with variety of plants as either saprotrophs or parasites, particularly in temperate regions.[2] Additionally, INA bacteria have been detected in aerosols, in precipitation and in the atmosphere.[3],[4] Limited ice nucleating bacterial species have been identified thus far. These include Pseudomonas syringae, P. viridiflava, P. fluorescens, Pantoea agglomerans, and Xanthomonas campestris.[2] Homology of INA genes and 16S ribosomal RNA sequence differences across these species suggest this trait was acquired through horizontal gene transfer from a common ancestor.[5]
Functionality
Molecular overview
Present in outer membrane of all ice nucleating bacteria,[1],[2] INA proteins (Ina) promote the alignment of water molecules for the formation of ice nuclei.[6] Ina has a hydrophobic N-termini with properties consistent with that of membrane-crossing domains and a hydrophilic C-termini which vary greatly across alleles.[2] The C-termini may be involved in protein folding.[1] Between the two is a large central core, the active site of ice nucleation.[2] Non-protein components of Ina, such as mannose and phosphatidylinositol, may have anchoring functions that may be important in efficient ice nucleation.[7] One model proposes that Ina monomers aggregate to form nucleation sites.[2] The sizes of these sites are positively correlated to nucleation frequency and temperature.[2]
Active Site of INA protein
The central core is the active site of Ina and acts as the template for interactions with water. The primary structure of INA protein consists of highly conserved repeats of four, eight, and forty-eight amino acids.[2] This periodicity acts as a template for ice formation.[2]The regular repeated sequence of amino acids in the central core permits consistent hydrogen bonding between the protein and neighboring water molecules.[2] One model by Kajava and Lindow proposes that these repeating sequences form a series of beta-hairpin secondary structures, as depicted in Figure 1.[6] These beta-hairpins interlock with one another, and this helps to stabilize the INA protein and adjoin areas of the hydrogen bonding template.[6] INA protein catalyzes ice formation by aligning water through template-like interactions through hydrogen-bonding and makes the ice nucleation more energetically favorable.[1],[2]
Expression of INA
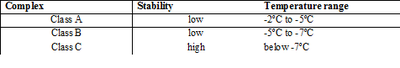
The expression and maintenance of ice nucleation activity is triggered by low environmental temperatures and nutrient starvation.[2] There are three classes of INA complexes, each optimized for specific environmental conditions as shown in Table 1.In a study by Rogers et al., Class A complex was observed to be up-regulated when the temperature was dropped from 30ºC to 5ºC, and down-regulated when the temperature was increased from 5ºC to 30ºC.[8] Furthermore, nutrient-starved INA bacteria have also been observed to up-regulate Class A complex.[9] This suggests that INA is a highly regulated process involving sensory of extracellular conditions.
Evolutionary Advantage of Trait
Ice nucleation enhances the ability of plant-associated bacteria to access nutrients under unfavorable environmental conditions and disperse over long distances. Firstly, ice nucleation and formation mechanically disrupts plant cells and provides the starved bacteria with nutrients leaked from damaged plant tissue.[2],[10] This also establishes a point of infection for pathogenic INA bacteria. Secondly, INA may help control and stabilize ice crystal formation and help the organism avoid membrane disruption.[2] Consequently, aerosolized INA bacteria may survive being carried by wind through the upper atmosphere and could potentially disseminate over long distances to new environments.[3] These factors could benefit the fitness of INA bacteria. =Application for detection of bacterial species
Agricultural Impact and Application
Bacterial ice nucleation can have both wanted and unwanted effects on vegetation. By stimulating ice formation in small quantities at higher temperatures, bacterial ice nucleation helps frost-tolerant plants avoid rapid and non-equilibrated freezing of its super-cooled fluids that could unevenly tear its tissues.[2] Conversely, bacterial ice nucleation can also cause frostbite in frost-sensitive plants, many of which are important agricultural products.[2] In this case, INA bacteria freeze plant cell fluids though the plant itself may tolerate mild sub-zero temperatures through super-cooling in the absence of INA bacteria.[2] Crops damageable by ice nucleating bacteria include wine grapes, olives, tea, apricots, and beans.[2] Several methods have been employed to limit frost damage caused by ice nucleating bacteria. These include the usage of bactericides, selective breeding of INA bacteria-resistant crops, and the employment of antagonistic non-ice nucleating bacteria to compete against ice nucleating bacteria.[1], [2]
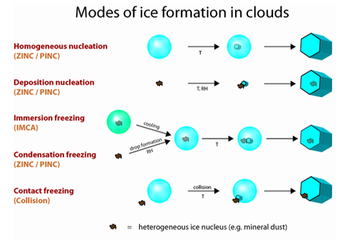
Cloud formation and precipitation
The involvement of INA bacteria in cloud formation through bioprecipitation has been proposed. Bacteria are emitted from terrestrial surfaces and are brought to the upper atmosphere by up-fluxes of air through storms and wind movements.[4] Agricultural plants, in particular, may be a major source of INA bacteria in the atmosphere.[2] For example, snap beans canopies were reported to emit 30 bacteria/m2/sec of INA positive strains of P. syringae.[2] Uplifted bacteria catalyze ice crystal formation, necessary for cloud formation and subsequent precipitation. This is suggested by detection of ice nucleating bacteria from many precipitants. For example, Raut et al. report INA bacteria in the core of hailstones,[11] Christner et al. detected ice nucleating bacteria in Antarctic snow samples,[12] and Constantinidou et al. report large downward fluxes of INA bacteria in rain.[3] However the significance of INA bacteria towards cloud-seeding is unknown. For example, Hoose et al. give 0.6% to be uppermost estimate of primary biological aerosol particles involvement in atmospheric ice nucleation.[13] There is yet to be substantial evidence suggesting that INA bacteria are significantly involved in cloud formation and precipitation since the involvement of INA bacteria in cloud formation is very difficult to experimentally validate due to difficult experimental design.[2]
References
(1) Gurian-Sherman D., Lindow S.E. “Bacterial Ice Nucleation: Significance and Molecular Basis.” FASEB J., 1993, 7:1338-1343.
(2) Morris C.E., Georgakopoulos D.G., Sands D.C. “Ice Nucleation Active Bacteria and Their Potential Role in Precipitation.” J. Phys. IV France, 2004, DOI:10.1051/jp4:2004121004
(3) Constantinidou H.A., Hirano S.S., Baker L.S., Upper C.D. “Atmosphereic Dispersal of Ice Nucleation-Active Bacteria: The Role of Rain”. Phytopathology, 1990, 80:934-937.
(4) Deleon-Rodriquez N., Lathem T.L., Rodriguez-R L.M, Barazesh J.M., Anderson B.E., Beyersdorf A.J., Ziemba L.D., Bergin M., Nenes A. “Microbiome of the upper troposphere: Species composition and prevalence, effects of tropical storms, and atmospheric implications”. PNAS, 2013, 110:2575-2580.
(5) Edwards A.R., Van Den Bussche R.A., Wichman H.A., Orser C.S. “Unusual Pattern of Bacterial Ice Nucleation Gene Evolution”. Mol. Biol. Evol., 1994, 11:911-920.
(6) Kajava A.V., Lindow S.E. “A Model of the Three-dimentional Structure of Ice Nucleation Proteins”. J. Mol. Biol., 1993, 232:709-717.
(7) Turner M.A., Arellano F., Kozloff L.M. “Components of Ice Nucleation Structures of Bacteria”. Journal of Bacteriology, 1991, 173:6515-6527.
(8) Rogers J.S., Stall R.E., Burke M.J. “Low-temperature conditioning of the ice nucleation active bacterium, Erwinia herbicola.” Cryobiology, 1987, 24:270-279.
(9) Fall A.L., Fall R. “High-level expression of ice nuclei in Erwinia herbicola is induced by phosphate starvation and low temperature.” Current Microbiology, 1998, 36:370-376.
(10) Lindow S.E. “The Role Of Bacterial Ice Nucleation In Frost Injury To Plants”. Ann. Rev. Phytopathol., 1983, 21:363-384.
(11) Raut A.A., Lokare S.S., Bajekal S.S., “Ice Nucleating Bacteria in Hailstones during Hailstorm over Karad, India.” Current Science, 2012, 103:990-991.
(12)Christner B.C., Morris C.E., Foreman C.M., Cai R., Sands D.C. “Ubiquity of Biological Ice Nucleators in Snowfall.” Science, 2008, 319:1214.
(13) Hoose C., Kristjánsson J.E., Burrows S.M. “How Important is Biological Ice Nucleation in Clouds on a Global Scale?” Environmental Research Letters, 2010, DOI:10.1088/1748-9326/5/2/024009.
