Baylisascaris procyonis and Associated Diseases
Introduction and Phylogeny
By Nadia Torok
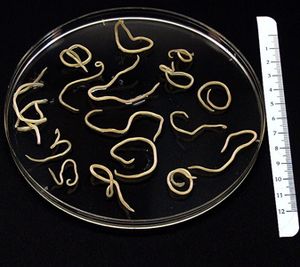
Baylisascaris procyonis is a parasitic nematode, or helminth, endemic to raccoon (Procyon lotor)[1] populations[2]. An image of adult B. procyonis worms can be seen at the right (Figure 1).Though raccoons are found throughout the United States, Baylisascaris procyonis-infected raccoons are most prevalent in the Midwest, the West Coast, the mid-Atlantic, and the Northeast[3]. Some sources say that up to 90% of adult raccoons in any given geographic area have been found to be infected with B. procyonis, and that the range of the parasite appears to be extending to include the southeastern United States as well[4].
The habitat of raccoons extends from Canada to Panama, meaning that raccoons, the definitive hosts for B. procyonis, are distributed widely across the United States[5]. Males, on average, weigh 6.76 kg, while females weigh an average of 5.94 kg[5]. They often live in wooded areas, where they construct dens[5] and establish latrine sites for defecation[3]. Raccoons eat both plant and animal matter, and often share habitat with skunks or opossums[5], which can also be infected by nematodes related to B. procyonis[2]. Raccoons are considered peridomestic, meaning they frequently have close contact with human populations[3], a trend that is increasing as humans further invade previously undeveloped habitats[6]. Despite the incredibly high prevalence of B. procyonis among raccoons, the animals display no symptoms upon infection[1].
Infection of humans by this parasite and the ensuing disorders are referred to as baylisascaris[2], and can affect other small mammals as well[1]. The species is distinguished from other helminthes by its invasion of the central nervous system (CNS) in hosts, as well as the continued growth of larvae within the CNS to a large size[2]. The first documented cases occurred in two young boys in 1984 and 1985[2].
This parasite is responsible for various manifestations of disease in humans and other animals, which can be serious and often fatal despite treatment[2]. Though infection with B. procyonis is rare—there have been fewer than 25 human cases reported in the United States[3]—infection is thought to be frequently misdiagnosed[3] and prognosis is very poor[2]. The major clinical manifestations of B. procyonis include visceral larva migrans (VLM), neural larva migrans, (NLM) and ocular larva migrans (OLM), which fall under the broader description of infection with B. procyonis, termed Baylisascaris[2].
Baylisascaris procyonis is a member of large nematodes in the order ascaridida, of the superfamily ascaridoidea[7], and is related to species such as Ascaris lumbricoides, Toxocara canis, and Toxocara cati, which are more commonly observed parasites affecting humans, dogs, or cats[2]. Originally, B. procyonis was classified as Ascaris columnaris in 1931, and it is closely related to Baylisascaris columnaris and B. melis, other worms that parasitize skunks and badgers[2]. A proposed phylogeny for Baylisascaris procyonis and related species can be seen below (Figure 2).
The mitochondrial genome is an emerging method of identifying species by genetic markers, particularly those, like B. procyonis, which possess high genome similarity to other species[8]. This may be beneficial in definitively diagnosing infection with Baylisascaris procyonis, which may otherwise be difficult[8]. The mitochondrial genome of B. procyonis is circular and contains 14,781 bp encoding 36 total genes, including 12 genes that code for proteins[8]. The mitochondrial genome of B. procyonis contains slightly higher AT composition than related species, at 70.5%[8]. Other nematodes range from 68.3%-72% AT content[8].
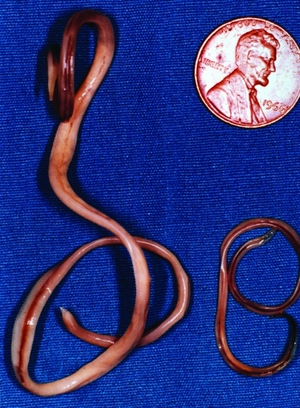
These roundworms are tan in color and exhibit sexual dimorphism, as females range in size from 20-22 cm and males from 9-11 cm[2], making them some of the largest parasitic nematodes[2]. An image of an adult specimen can be seen at the left (Figure 3). Females lay 115,000 to 179,000 eggs per day[2]. Eggs are an ellipsoidal shape, dark brown, and 63-88 μm by 50-70 μm in size[2]. It is estimated that raccoons shed up to 20,000 eggs per gram of feces[1], while only 5,000 eggs or fewer is sufficient to infect a human[9]. An image of male and female adult B. procyonis obtained from a raccoon can be seen at the left.
Life Cycle
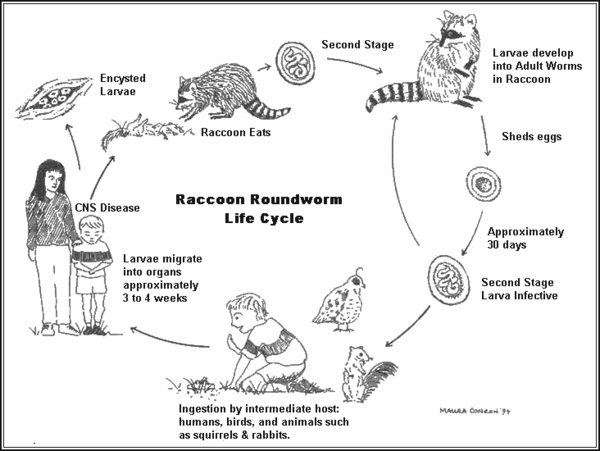
Raccoons are usually infected with B. procyonis while foraging or feeding, though they may also become infected after grooming other raccoons whose fur contains B. procyonis eggs or by eating other animals infected with roundworm larvae[3]. Juvenile raccoons appear to be infected more frequently than adult raccoons with a prevalence of 93.5% in juveniles compared to 55.3% prevalence in adults[2]. This is thought to be because larvated eggs, found on fur and in feces, can cause infection in juveniles, while adults can only become infected after ingesting an intermediate host, such as the carcass of a small mammal, that has been infected[6]. A diagram of the life cycle of Baylisascaris procyonis can be seen below (Figure 4).
A major concern in the transmission of B. procyonis is its potential to be passed on in the feces of raccoons coupled with the tendency of raccoons to defecate in public areas, called latrines[3]. Latrines are sites where raccoons or groups of raccoons repeatedly defecate, and they usually contain approximately 370-750 g of feces[2]. In a study of latrine structure and its role in transmission, Page et al. found that up to 68% of latrines containing B. procyonis eggs were not in current use by raccoons[1], and stated that the preference for latrine sites on logs or beneath large trees was concerning because these areas are often along travel routes or foraging paths for small animals, whose populations could be impacted by infection with B. procyonis[1]. Furthermore, the negative impacts on populations of small invertebrates are potentially widespread owing to the nonspecificity of infection by B. procyonis[1]. Dogs, as alternate hosts for B. procyonis, could also contribute to the spread of the parasite by shedding eggs in their feces, particularly in residential areas[10].
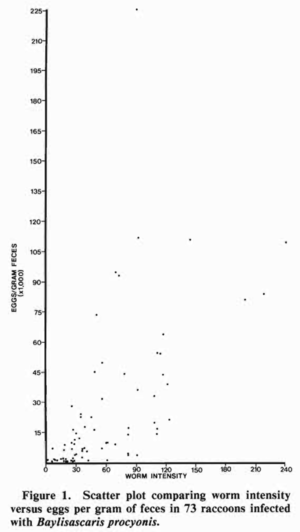
Once excreted by raccoons, the unembryonated eggs are not immediately infectious to humans or other hosts; however, they are highly robust, and are able to survive for years, even in harsh conditions[3][1]. It is estimated that eggs have the ability to survive for at least 5-6 years outside a host[1]. At right is a graph relating worm intensity and the amount of eggs per gram of feces, used to define “worm prevalence” (Figure 5)[11]. Worm prevalence has been used as a measurement to describe trends among raccoons according to age, sex, or other factors[11]. It can be seen from the figure that worm intensity increases as the number of eggs per gram of feces increases. Snyder and Fitzgerald conducted one of the earliest studies of B. procyonis in which they measured the prevalence of the parasite in the fecal samples from 100 raccoons[11]. The study investigated both worm prevalence and worm intensity, and found that juvenile raccoons had a higher prevalence of worm prevalence than adults, though they were comparable in all other measurements[11]. Their results indicated a higher intensity of B. procyonis than had been observed in previous studies, and was the first to examine differences due to age[11]. A surprising result Snyder and Fitzgerald encountered was that the fecundity of the worms present in adult raccoons was equal in both juvenile and adult raccoons, though the higher worm intensity of juveniles resulted in more egg shedding overall[11]. Finally, Snyder and Fitzgerald pointed out that worm prevalence, intensity, and fecundity are likely to be affected by a number of factors, including the number of worms present, physiological conditions within the host, and the age of the infecting worms[11].
After 2-4 weeks, the eggs become embryonated and therefore infective, at which point they may be consumed by small animals, birds, or humans[3]. In these alternate hosts, or “transport hosts,” worms do not develop fully into adult worms; rather, larvae remain encysted (encased in tissue)[12] until ingested once more by a raccoon[13]. Infection may ensue after 4-5 weeks, and is difficult to eradicate[4]. The embryonated eggs then hatch and the larvae migrate through the gut wall to other tissues and develop into adult worms in the small intestine[3]. In the human gut, this migration can be a source of various afflictions of the central nervous system[4], a trait unique to the B. procyonis species.
The migration of B. procyonis larvae is a pattern of parasitosis referred to as larva migrans, and the raccoon roundworm is in fact the most common source of larva migrans in animals[2]. Other forms of larva migrans can occur as larvae migrate through various tissues; in particular, we will focus on ocular larva migrans (OLM), neural larva migrans (NLM), or visceral larva migrans (VLM) as they occur in humans[2].
Larva Migrans
Invasion of tissues by larvae during larva migrans causes an inflammatory immune response in the host, stimulated by release of an excretory-secretory antigen in humans and resulting in damage to affected tissues[12]. Larva migrans manifests differently as the result of infection by different organisms. In the related species Toxocara spp., for example, visceral larva migrans, toxocariasis, and ocular larva migrans occur, but not neural larva migrans[12]. These conditions typically do not occur simultaneously in Toxocara-induced larva migrans[12], but frequently coincide during Baylisascaris-induced infection[14]. Sensitive organs and the central nervous system are the primary targets of visceral larva migrans, which can damage the liver, lungs, heart, bowels, and lymph nodes[12], resulting in hepatitis or pneumonitis and most commonly affecting children under three years of age[12]. Seizures can occur if the central nervous system is breached[12].
In contrast to larva migrans caused by Toxocara, visceral larva migrans that occurs upon infection by B. procyonis usually accompanies the other manifestations of larva migrans[15]. The symptoms of visceral larva migrans are often predominated by the more severe symptoms neural larva migrans, which can additionally coincide with ocular lesions from migration of worms to the eyes (ocular larva migrans)[14]. Symptoms of visceral/neural larva migrans appear suddenly after as little as 2-4 weeks post-infection, and may include lethargy, irritability, weakness, and loss of motor skills and head control[14]. Initially, a rash may be observed on the face and chest[12]. As the disease progresses, victims may lose their vision and ability to speak, eventually falling into a coma, followed by death[14]. The severity of symptoms is dependent on how many worms were ingested, as well as the extent and location of migration[16].
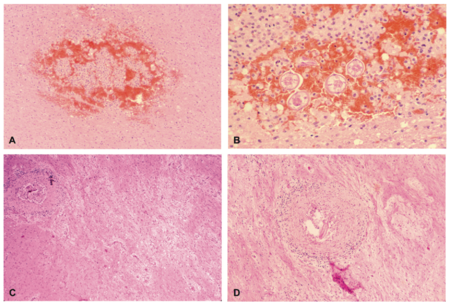
Similarly to infection with Toxocara, infection with B. procyonis is most prevalent among young children[14][12], making the severe and permanent cognitive and neural impairments[14] resulting from infection even more devastating. These effects may include incontinence, seizures, paralysis, and blindness[14][12]. Upon autopsy, larvae have been located within the cerebrum, cerebellum, and spinal cord, and their presence is often accompanied by inflammatory reaction[2]. Other results of infection include necrosis and evidence of an immune response, such as macrophages, eosinophils, and lymphocytes[2]. The images on the left (Figure 6) show examples of tissues damaged by fatal B. procyonis infection in a monkey (A, B) and a child who died of the infection (C, D).
Higher prevalence among infants, children, and the developmentally delayed is thought to be due to the practices of pica and geophagia (tendencies to eat substances, such as dirt, that are not considered food), as well as unformed hygiene habits[14][12]. These behaviors put individuals at a much higher risk of accidentally ingesting raccoon fecal material containing eggs. In addition to displaying increased risky behaviors, such as pica and geophagia, children are more likely to be severely affected by NLM due to the smaller size of their brains, which increases the severity of damage caused by migrating larvae due to the decreased number of worms necessary to damage the smaller amount of tissue[12].
Diagnosis of B. procyonis neural and visceral larva migrans can be challenging. Unlike with many parasitic afflictions, an examination of the patient’s stool for ova and parasites is not useful because of the absence of adult worms during human infection and thus a lack of eggs in the stool[16]. In adults, the presence of larvae is often much lower than that found in children, and serology tests may give negative results[14]. However, identification of larvae is considered a confirming diagnosis[14]. Other tests that have been used include indirect immunofluorescence, western blotting, and a larval excretory-secretory antigen[14]. Neuroimaging tests, electroencephalography, and brain biopsies have also been utilized[14]. Primarily, diagnosis is the result of ruling out infection by related organisms causing similar symptoms[9].
Another major manifestation of B. procyonis is ocular larva migrans, in which the eye or the optic nerve is invaded by the parasite[12][14][17]. A single larva is sufficient to cause OLM[14]. Ocular larva migrans is observed in adults, unlike neural larva migrans, which is almost always seen in infants and young children[12]. Males appear to be affected more frequently than females[17]. Upon infection by Toxocara, the sources of vision loss are grouped into three main categories: intraocular inflammation, retinal comorbidities (co-occurring risk factors), or damage as a result of the immune response-induced inflammation and encapsulation of the larvae (forming a granuloma) itself[17]. Migrating larvae are thought to damage tissues by releasing excretory-secretory products, such as enzymes or waste products[14].
Onset of OLM is marked by a sudden decrease in vision in a single eye[14]. Identification of ocular larva migrans can be made by ophthalmologic examination and/or serum antibody tests revealing antibodies to the parasite larvae[17][14]. Larvae morphology allows physicians to determine whether B. procyonis or Toxocara is the cause of OLM[2]. B. procyonis larvae are much larger than Toxocara larvae, which are 350 to 445 µm by 20 µm[2]. Toxocara and Ancylostoma are much more common sources of OLM than B. procyonis[2][14].
Treatment and Prognosis
Infection by B. procyonis has a poor prognosis, regardless of its manifestation as OLM, NLM, or VLM[12]. As of 2005, it was reported that all survivors of NLM were living in a persistent vegetative state or requiring full-time nursing care due to severe deficits[12]. Even use of anthelmintics is unsuccessful in most cases[12]. Though Anthelmintics can be effective in eradicating intestinal worms in raccoons and skunks, they are much less effective against human infection[2]. Common anthelmintics used against the related Toxocara canis species, found in dogs, are sometimes used against B. procyonis, though with little success in humans[4]. Among common anthelmintics, thiabendazole, fenbendazole, tetramisole, and ivermectin are all ineffective against B. procyonis[2].
Newer studies are hopeful that albendazole and diethylcarbazine work well, as they demonstrate good penetration of cerebral spinal fluid and larvicidal activity[2]. Albendazole is preferred due to its better absorption, permeation of the blood-brain barrier, and low toxicity[2]. Experiments with mice revealed that treatment is much more effective when started as early in the course of infection as possible[2]. Prophylactic treatment is under consideration for children with known exposure to raccoons or raccoon feces, and shows promise for preventing lasting harm or fatalities[2].
In OLM, laser surgery has been effective in killing the larvae, but the pre-existing tissue damage and vision loss are irreversible[9]. Other, more common, therapies focus on treating inflammation, usually with a combination of glucocorticoids and corticosteroids[12]. Almost invariably, vision loss is a result of ocular larva migrans, particularly with B. procyonis[14].
Treatment of worms in raccoons and dogs, an alternate host of B. procyonis, has been very successful[10]. Treatment of dogs is a priority because the prevalence of B. procyonis in dogs is believed to be increasing, and the adaptation of the parasite to canines, resulting in easier infection of canines, is a concern due to the proximity of humans and their canine companions, leading to an increased risk of transmission if dogs are more readily infected[10]. Various different anthelmintics have been shown to completely clear raccoons of parasites, and trials using milbemycin oxime on dogs also resulted in clearing of intestinal worms in the majority of subjects[10]. Routine deworming of dogs is recommended by vets to prevent symptoms of infection such as mild diarrhea or intestinal obstruction[4], though treating their canine companions would also benefit humans.
Current Studies and Remaining Uncertainty
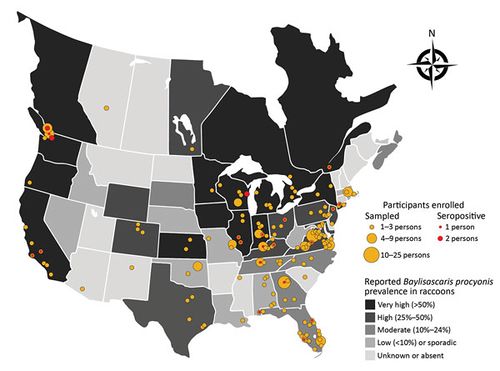
Just how concerned should we be about infection with B. procyonis? The prevalence of underdiagnosis is difficult to ascertain, but various studies provide insight into the patterns of transmission of this infection and possible methods of decreasing risk for humans and other hosts. It is possible that many people may be carrying B. procyonis without displaying any symptoms, referred to as “subclinical” infection[18]. Toxocara seropositivity has been encountered in approximately 14% of adults, indicating a potentially similar pattern for Baylisascaris procyonis[18]. Wildlife rehabilitation workers, who have frequent contact with raccoons or other animals that may be carrying the disease, are a population likely to have high incidence of seropositivity, leading Sapp et al. to conduct a study to determine the prevalence of antibodies against B. procyonis in workers with high contact with wildlife (Figure 7)[18]. As would be expected, this figure shows that higher prevalence of Baylisascaris procyonis tends to align with higher incidence of seropositivity in areas across the United States and Canada, ranging from Florida to Washington to Quebec[18]. Antibodies were found in 7% of participants, who all had documented risk factors, including handling raccoons and inconsistent glove use[18]. These results indicate a potential for infection by B. procyonis without clinical disease, a previously unanticipated result[18]. These results are consistent with the high prevalence (up to 14%) of subclinical infections by related species, such as Toxocara; however, it is important to note that the population in the study had already been identified as a group at high risk for exposure to B. procyonis[18].
Though the consequences of infection with B. procyonis are alarming, infections causing sufficient symptoms for a diagnosis are extremely rare, with only 12 cases reported and several more suspected since the first reported case in 1985[16]. There remains concern, however, that increased migration of humans into raccoon habitat will lead to an increase in the prevalence of baylisascarisis and other zoonotic diseases[15].
The potential effects of B. procyonis infection on wildlife conservation efforts are more of a concern than the spread of the parasite among humans, which occurs at a low rate[19][16]. Wildlife is at risk due to the latrine habits of raccoons and the broad range of potential hosts for B. procyonis. Small mammals, particularly granivores, are most likely to be affected, due to their foraging habits[20]. In addition to the neurological damage similar to that produced in humans, behavioral changes such as circling or lack of coordination may make infected animals easy prey, thus leading to further transmission to predators[20]. Animals that tend to forage through fresh fecal matter for seeds are more likely to ingest undeveloped eggs than animals that forage through old fecal matter; therefore, foragers of fresh feces are less likely to develop symptoms of infection[20].
Other factors in transmission of B. procyonis include increased host-to-host contact due to anthropogenic disturbances[19]. In suburban areas, it is suggested that limiting access to resources provided by humans, such as food, would help decrease the “clumping” of raccoon populations and therefore decrease population density and associated transmission rates[19]. Other efforts at preventing the spread of B. procyonis include trapping and relocating raccoons or destroying the eggs by means of boiling lye water, boiling Lysol, or a propane torch[15].
Though B. procyonis infection is generally believed to correlate with population density[19], another study has shown that populations of raccoons in urban locations were found to exhibit a lower prevalence of infection with B. procyonis, as well as decreased worm burdens, indicative of infection intensity, in comparison with raccoons collected from rural environments[6]. This was in contrast to the expected hypothesis that rural raccoon populations would exhibit a lower prevalence of infection due to lower population density[19][6]. It is thought that rural populations of raccoons rely more heavily on predation for a food source, whereas urban populations frequently consume food from human sources, such as unprotected trash receptacles[6]. Because intermediate hosts that are often prey to raccoons contribute to transmission of B. procyonis, the decreased reliance on predation is cited as an explanation for the decreased effects of the parasite observed among urban populations[6]. Thus, it is less clear what actions humans should take to decrease the prevalence of B. procyonis among raccoons, making it imperative that preventative action be taken to prevent infection of humans themselves.
References
I would like to thank E.R. Rovani, a dear friend and inspirational microbiologist, for informing me about Baylisascaris procyonis. Good times...Raccoon!
-N. Torok
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Page, K.L., Swihart, R.K., and Kazacos, K.R. "Raccoon Latrine Structure and Its Potential Role in Transmission of Baylisascaris procyonis to vertebrates." 1998. The American Midland Naturalist 140(1):180-185. Cite error: Invalid
<ref>tag; name "Page" defined multiple times with different content - ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 2.26 2.27 2.28 Gavin, P.J., Kazacos, K.R., and Shulman, S.T. "Baylisascariasis." 2005. Clinical Microbiology Reviews 18(4): 703-718. DOI: 10.1128/CMR.18.4.703-718.2005
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 "Parasites - Baylisascaris Infection." 2012. Centers for Disease Control Global Health - Division of Parasitic Diseases.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Baylisascaris procyonis" 2015. Companion Animal Parasite Council.
- ↑ 5.0 5.1 5.2 5.3 Lotze, Joerg-Henner and Anderson, Sydney. "Procyon lotor." 1979. American Society of Mammologists 119:1-8.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Page, L. Kristen, Gehrt, Stanley D., and Robinson, Nathaniel P.. "Land-use effects on prevalence of raccoon roundworm (Baylisascaris procyonis)." 2008. Journal of Wildlife Diseases 44(3):594-599. DOI: 10.7589/0090-3558-44.3.594
- ↑ Bauer, Christian. "Baylisascariosis--Infections of animals and humans with ‘unusual’ roundworms." 2012. Veterinary Parasitology 193: 404-412. DOI: 10.1016/j.vetpar.2012.12.036
- ↑ 8.0 8.1 8.2 8.3 8.4 Xie, Yue, Zhang, Zhihe, Niu, Lili, Wang, Qiang, Wang, Chengdong, Lan, Jingchao, Deng, Jiabo, Fu, Yan, Nie, Huaming, Yan, Ning, Yang, Deying, Hao, Guiying, Gu, Xiaobin, Wang, Shuxian, Peng, Xuerong, and Yang, Guangyou. "The mitochondrial genome of Baylisascaris procyonis." 2011.PLoS ONE 6(10):e27066. DOI:10.1371/ journal.pone.0027066
- ↑ 9.0 9.1 9.2 Southern Nevada Health District. "Raccoon Roundworm (Baylisascaris procyonis)." 2007. Southern Nevada Health District, Office of Epidemiology. Cite error: Invalid
<ref>tag; name "Nevada" defined multiple times with different content - ↑ 10.0 10.1 10.2 10.3 Bowman, Dwight D., Ulrich, Michael A., Gregory, Dawn E., Neumann, Norwood R., Legg, Walter, and Stansfield, David. "Treatment of Baylisascaris procyonis infection in dogs with milbemycin oxime." 2004. Veterinary Parasitology 129:285-290. DOI: 10.1016/j.vetpar.2004.02.030
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Snyder, Daniel E., and Fitzgerald, Paul R. "Contaminative potential, egg prevalence, and intensity of Baylisascaris procyonis-infected raccoons (Procyon lotor) from Illinois, with a comparison to worm intensity ." 1987. Proc. Helminthol. Soc. Wash. 54(1):141-145.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 12.14 12.15 12.16 12.17 Hare, Anna Quinn, and Franco-Paredes, Carlos. "Ocular Larva Migrans: A Severe Manifestation of an Unseen Epidemic." 2014. Current Tropical Medicine Reports 1(1): 69-73. DOI: 10.1007/s40475-013-0004-5
- ↑ Junquera, P. " Baylisascaris procyonis, the raccoon roundworm, parasite of dogs." 2015. Parasitapedia.net
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 14.11 14.12 14.13 14.14 14.15 14.16 14.17 14.18 Kazacos, Kevin R., Jelicks, Linda A., and Tanowitz, Herbert B. "Baylisascaris larva migrans." 2013. Handbook of Clinical Neurology: Neuroparasitology and Tropical Neurology. 113: 251-262. DOI: 10.1016/B978-0-444-53490-3.00020-0
- ↑ 15.0 15.1 15.2 Wise, Matthew E., Sorvillo, Frank J, Shafir, Shira C., Ash, Lawrence R., and Berlin, O. George. "Severe and fatal central nervous system disease in humans caused by Baylisascaris procyonis, the common roundworm of raccoons: a review of current literature." 2005. Microbes and Infection 7(2):317-323. DOI: 10.1016/j.micinf.2004.12.005
- ↑ 16.0 16.1 16.2 16.3 Strausbaugh, Larry J., Murray, William J., and Kazacos, Kevin R. "Raccoon roundworm encephalitis." 2004. Clinical Infection Diseases 39(10):1484-1492. DOI: 10.1086/425364
- ↑ 17.0 17.1 17.2 17.3 Ahn, Seong Joon, Ryoo, Na-Kyung, and Woo, Se Joon. "Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention." 2014. Asia Pacific Allergy. 4:134-141. DOI: 10.5415/apallergy.2014.4.3.134
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 Sapp, Sarah G.H., Rascoe, Lisa N., Wilkins, Patricia P., Handali, Sukwan, Gray, Elizabeth B., Eberhard, Mark, Woodhall, Dana M., Montgomery, Susan P., Bailey, Karen L., Lankau, Emily W., and Yabsley, Michael J. "Baylisascaris procyonis roundworm seroprevalence among wildlife rehabilitators, United States and Canada, 2012-2015." 2016. Emerg Infect Dis. 22(12):2128-2131. DOI: 10.3201/eid2212.160467
- ↑ 19.0 19.1 19.2 19.3 19.4 Matthew E., and Wright, Amber N. "Altered prevalence of raccoon roundworm (Baylisascaris procyonis) owing to manipulated contact rates of hosts." 2005. J. Zool., Lond. 266:215-219. DOI: 10.1017/S0952836905006813
- ↑ 20.0 20.1 20.2 Logiudice, K. "Latrine foraging strategies of two small mammals: Implications for the transmission of Baylisascaris procyonis." 2001. The American Midland Naturalist, 146(2):369-378.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
