Brevibacillus laterosporus, a bacterial biological control agent of Western Corn Rootworm
Background
By Katya Naphtali
Underneath the umbrella of pesticides, there are biologically synthesized alternatives to chemicals called microbial pesticides. These alternatives use different microorganisms to target specific pests that threaten a given crop production. The actual material used as a pesticide is naturally formed, biodegradable, and highly specialized for specific pests rather than having a widespread environmental impact. Additionally, microbial pesticides can be used in small quantities with large impacts [1]. This technique of pest control is commonly used to address damage incurred by the Western Corn Rootworm (CRW), which is specialized to parasitize corn plants [2]. Damage incurred from CRW infestation of corn crops costs the United States a total of $2 billion annually [3].
A common management process to address CRW is the incorporation of microbial pesticides into corn GMO species. A number of crops including cotton, soybeans, and corn are genetically engineered to contain gene sources from the soil bacteria, Bacillus thuringiensis, commonly referred to as "Bt". This species is most commonly used for microbial pest control targetted at Coleopteran species [4]. The toxins produced by Bacillus bacteria attack cells in the larva’s midgut by causing the cells in their stomach lining to lyse [5]. When genes for toxic protein production are incorporated into the genetic material of the crop itself, the pests that parasitize these crops die from consuming these naturally synthesized toxins [4]. By producing these toxins, GMO corn is both protected from CRW cause damage and reduces the overall larval population of CRW in a given field. Currently, there are four strains of Bt corn that are widely incorporated into corn productions [5].
Unfortunately, in some regions where these Bt toxins are overused, CRW has adapted resistance to these toxins [2][6]. Currently, there are populations of CRW that have evolved to be resistant to every strain of Bt we have documented currently (in April of 2023) [5]. This drives researchers to search for similarly effective bacteria to replace their current technology.
Diabrotica virgifera virgifera, Western Corn Rootworm
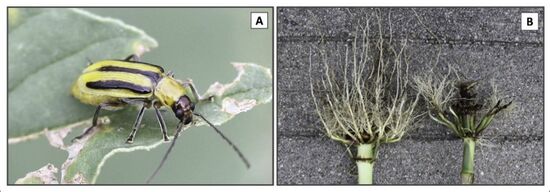
Diabrotica virgifera virgifera, otherwise known as Western Corn Rootworm, is a species of Coleoptera (beetle order) that specialize only in eating varieties of corn, Zea mays. These beetles are ¼” long and yellow with black stripes along the sides of their elytra. They only have a single generation a year, because their life cycle is dependent on corn developmental stages [7]. Adult beetles lay their egg clusters below the soil surface to produce the next generation, before dying themselves in the first winter frost [2]. These eggs hatch during late May and early June into polypod larvae that are long small and white and sport a sclerotized brown head plate with strong mandibles. They spend most of their larval stage feeding on the root zone of the soil. They tunnel through the soil until finding corn roots to feed on [7]. This tunneling can kill the entire root or break the tips off, this prevents the plant from receiving enough nutrients causing it to fall over, or greatly damage growth. The plant can be weakened in terms of stunting growth, reducing the yield amount, or even reducing the size of each ear of corn [2].
These larvae undergo three instars of molting over a 4-6 week period [8][7]. Before they pupate within the soil to undergo a complete metamorphosis of their bodies into their adult forms. 5-10 days after pupation, CRW emerge as adults coinciding with when the corn plants flower [8][7]. CRW beetles still consume food during their adult stages, mainly corn pollen and corn silk, and sometimes corn leaves as well. Corn silk and pollen are both needed for corn pollination, so parasitism by adult CRW reduces corn pollination ability [2]. The negative impact of adult parasitism still causes less damage than the damage caused during the larval stage feeding on corn roots [9].
In laying their eggs for the next generation, adult CRWs are relatively stagnant species and do not tend to move fields for laying their eggs. As a result, a common pest management technique for CRW is crop rotation with soybeans [5]. Switching corn out for other crops yearly causing CRW larvae populations to drop due to the lack of their niche corn food source. Unfortunately, in some cases, CRWs have adapted to crop rotation schedules and have begun laying their eggs in soybean fields in anticipation of crop rotation to corn the following Spring when their offspring will hatch [2]. CRWs adapt quickly due to their dependence on corn as their sole food source [10]. As a result, other forms of pesticides are needed to be used alongside crop rotation [2].
Most commonly, crop rotation is paired with the use of Bt corn crops, that have been genetically modified to produce these pest-specific toxins themselves. These genetic modifications allow farmers to use fewer chemical pesticides while still reducing the damage incurred by pests, and increasing their overall crop yield [4]. Unfortunately, Bt is widely used which has resulted in the emergence of multiple resistant strains of CRW both in Northwest Iowa and Southeast Minnesota. While these resistant populations may not be able to move far without accidental human transportation, they foretell the immunity that may be seen in other regions that over-use specific resistant strains. As a result, new bacterial strains are being investigated to create new resistant corn GMO archetypes. Similar to crop rotation, a rotation of toxins may introduce more variety in pest control techniques that will reduce their ability to adapt to each individual tool [2].
Brevibacillus laterosporus, Bl
A number of Bacillus species have been investigated for pesticidal properties due to their formation of specialized biotoxins. Since the 1960s the most commonly used biotoxin has been those sourced from various strains of Bt. These bacteria are produced industrially in fermentation tanks and have widespread use for various different orders and species of insects [11]. As pest species become more resistant to Bt toxins, researchers have taken interest in a different but similar species of Bacillus, Brevibacillus laterosporus. In recent years Brevibacillus laterosporus has become a bacterial species of more interest due to the speed in which it produces biotoxins, even if less powerful of a toxin than those Bt could produce [12][13].
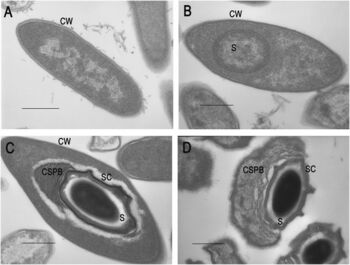
Brevibacillus laterosporus (Bl) is a gram-positive, rod-shaped bacteria about 3-5um in length and 0.7-1.0um in width. This bacteria is facultatively anaerobic [14] meaning Bl can survive in environments with or without oxygen. It also can either be found individually or in short chains [15] which can form flat white colonies [12]. Due to its metabolic flexibility, Bl can be sourced from soil, plants or even directly from insects themselves [12]. These bacteria reproduce by producing endospores. On one end of each spore, this species of bacteria can be identified by its production of canoe-shaped parasporal bodies (a crystallized shell)[12]. Bl produces a number of products including enzymes and prebiotic materials that function as an insecticide [16]. Some of these insecticidal products are sourced from the crystallized structures of these parasporal bodies that contain endotoxins [12]. To extract these endotoxins, during cell lysis, parasporal bodies detach from the cell wall and can be removed as a precipitate. This material can then be utilized as a larvacide. Different strains of Bl produce different shapes and structures of proposal bodies but their functional differences are unclear [17]. Additionally, some strains of Bl do not even form proposal bodies, but still are larvicidal [13]. While there are still many unknowns, Bl produces parasporal bodies and endotoxins at a higher rate than other species of Bacillus making it optimal for industrial agricultural use [12].
Larvicidal Properties of Bacillus
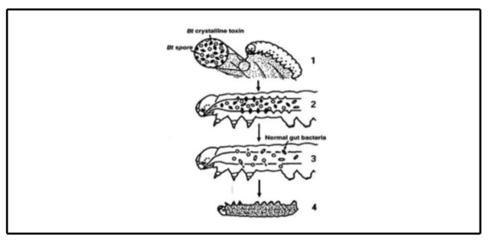
Since Bt has been used nationally since the 1960s [11] there is more information on its role as an insecticide than for Bl. The endotoxins produced by Bt are fast-acting, minutes after target insects consume Bt treated leaves, its endotoxins bind to receptors in the pest’s gut. These receptor sites in the gut wall are specific to toxins and species, allowing this form of pesticide to be highly specialized to either insect order or a handful of species. Once bound to this receptor site, over 1-2 hours this toxin begins lysing the wall of the gut. Once the gut wall is broken down, the toxin has already dissolved and spores from the Bt bacteria along with microbes from the insect’s own digestive system leak out into the rest of their body, and into their hemolymph. Once infected, the host insect stops eating and the insect dies within 1-2 days from septic infection in the blood [11]. Bl endotoxins work similarly through the gut of their target organisms, but less is known about how lethal it is compared to Bt. Initially sourced strains of Bl were collected from water sampling in 1916 [18] and these initial samples happened to have lower insecticidal abilities [15]. As a result, studies comparing Bl to Bt strains assumed that Bl had weaker larvacidal abilities than Bt, and that Bl also had a more limited environmental range [13].
In more recent years, more strains have been identified using 16S RNA sequencing [15] These strains have been found to function as a broad spectrum antimicrobial agents with lethal impacts on Coleoptera as well as Lepidoptera, Diptera, and nematodes. With no pathogenic effect on non-target species [17]. Bl is toxic to larvae only through ingestion which allows the endotoxins to bind to their gut wall receptors [17][11] This toxin kills pests swiftly and dissolves within their gut in a matter of hours[11]. As a result, to effectively use Bl as a biocontrol, its genes need to be used in the creation of GMO crops or as a topical insecticide. For topical application,
Bl mixtures must coat the entire crop plant, especially the undersides of leaves, due to the fact that it is deactivated by ultraviolet light from direct sunlight.
MORTALITY RATE OF Bl
What has been found is that Bl has an almost 100% effectiveness rate in the elimination of Coleopteran order pests depending on the strain. It was most effective in targeting beetles during their larval stage, especially during their second instar [19].
A different study found that Bl causes a 33-63% mortality rate in the larva of Coleoptera, but up to a 90% mortality rate results on caterpillars (Anticarsia gemmatalis). Regardless of species, toxins had the strongest effects when collected during exponential growth phases which incurred a mortality rate of 90% compared to the lower range of toxins sourced during vegetative growth stages at 13-48%[13]. Lastly, the lethal values of each bacteria are similar between Bt and Bl which indicates that Bl may be just as lethal as Bt it utilized correctly[16].
Specific Properties of B. laterosporus
INSECTICIDAL PROTEINS (ISPs)
Bl produces proteins that are similar to those used from Bt. Specifically, Bl secretes insecticidal proteins (ISPs) into their surrounding environment, called the broth. From this broth, we can collect a supernatant of isolated ISPs that is highly toxic to CRW [17]. These ISPs include two types, ISP1A and ISP2A. These proteins are only toxic when consumed together with a complementary protein, they do not hold toxic properties individually. This protein combination has similar lethal effects on larva homologous to the ISPs produced by Bt during a vegetative growth stage [17].
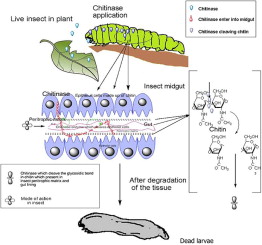
CHITINASE PRODUCTION
When this bacteria is in the presence of chitin, it produces extracellular chitinase to break it down outside of the cell in order to convert it into a carbon source it can metabolize within the cell [15]. Chitin is a polysaccharide that can be found in the body structure of insects, used to strengthen exoskeletons [20]. Chitin cannot be found in other organisms such as humans, plants, and other insects making this a more targeted insecticide than chemicals that may have destructive impacts on the surrounding environment [15]. These chitinases can be removed from the bacteria and turned into a mixture used as an insecticide. Bl already possesses insecticidal toxins, through the formation of proposal bodies, but in the presence of chitin, it has an additional toxin source [15]. While the Bl bacteria itself is adapted for moderate temperatures, surprisingly the chitinase sourced from it can remain active at up to 70oC and are more thermostable than predicted. This would indicate that chitinase mixtures sourced from Bl are well suited for industrial production as they would still be effective in conditions and environments different from their adapted ecosystem [15].
When this chitinase mixture is sprayed on leaves experimentally, this mixture had a faster mortality rate than bacteria that were not pre-exposed to chitin [15]. When the larva of diamondback moths (Plutella xylostella) were exposed to Bl with a chitinase mixture, 50% of the larva died within 2.1 days compared to without the mixture in which it took 3.3 days to reach a 50% mortality rate. The use of chitinase made the mortality rate 1.2 days shorter. This could be explained due to how chitin targets and weakens the inner gut lining of insects [21], both degrading the gut directly and allowing proposal body toxins to penetrate the gut as well [15][22].
Prassana et al. (2013)[15] studied this by inducing chitinase production in some Bl bacteria and collected proteins. Then they looked at the impact of chitinase applied to un-induced bacteria to see if it had an additive effect on the insecticidal properties of Bl. This chitin culture serum increased the lethal effect of Bl application on leaves with a dosage effect. With a 500ul dose, 100% of the larva died within 5 days. With every other dosage, or condition without chitinase, 100% larvae were still eliminated within 7 days [15]. They found that mortality was higher during the first instar while the 5th had the lowest mortality rate when exposed to chitinase. This may be due to the high feeding rates at earlier developmental stages as opposed to later instars where moths feed less and thus have less exposure to the insecticides spread on the leaves[15].
Conclusion
Brevibacillus laterosporus holds the potential to be an effective specialized larvicide. More research needs to be conducted on the specific impacts Bl may have on Western Corn Rootworm as well as creating variety in microbial pest control and variation similar to that of crop rotation. In combination with other IPM techniques, Bl could serve as an effective tool for managing Coleopteran pest species. While this strain is not as powerful as Bt, it holds the potential to be as lethal to pests, and due to its low usage in agriculture thus far, few pests will be resistant to its toxins.
References
- ↑ US EPA. 2022, “What are Biopesticides?” United States Environmental Protection Agency.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Cornell. “Northern and Western Corn Rootworms” Cornell University College of Agriculture and Life Sciences.
- ↑ Wechsler, Seth, and David Smith. “Has Resistance Taken Root in U.S. Corn Fields? Demand for Insect Control.” American Journal of Agricultural Economics 100, no. 4 (2018): 1136–50.
- ↑ 4.0 4.1 4.2 Fernandez-Cornejo, Jorge, and William D McBride. “Genetically Engineered Crops for Pest Management in U.S. Agriculture: Farm-Level Effects,” U.S. Department of Agriculture. Agricultural Economic Report No. 786.
- ↑ 5.0 5.1 5.2 5.3 Paddock, Kyle J., Christelle A. M. Robert, Matthias Erb, and Bruce E. Hibbard. “Western Corn Rootworm, Plant and Microbe Interactions: A Review and Prospects for New Management Tools.” Insects 12, no. 2 (February 17, 2021): 171.
- ↑ Levine, Eli, Joseph Spencer, Scott Isard, David Onstad, and Michael Gray. “Adaptation of the Western Corn Rootworm to Crop Rotation: Evolution of a New Strain in Response to a Management Practice.” American Entomologist 48 (April 1, 2002): 94–107.
- ↑ 7.0 7.1 7.2 7.3 Strnad, S. P., and P. E. Dunn. “Host Search Behaviour of Neonate Western Corn Rootworm (Diabrotica Virgifera Virgifera).” Journal of Insect Physiology 36, no. 3 (January 1, 1990): 201–5.
- ↑ 8.0 8.1 Corn Rootworm IPM Extension. 2023. “Corn Rootworm Life Cycles” Iowa State University of Science and Technology.
- ↑ Godfrey, L. D., L. J. Meinke, R. J. Wright, Affects of Larval Injury by Western Corn Rootworm (Coleoptera: Chrysomelidae) on Gas Exchange Parameters of Field Corn, Journal of Economic Entomology, Volume 86, Issue 5, 1 October 1993, Pages 1546–1556.
- ↑ Rausher, Mark D. “Co-Evolution and Plant Resistance to Natural Enemies.” Nature 411, no. 6839 (June 2001): 857–64.
- ↑ 11.0 11.1 11.2 11.3 11.4 Weinzierl, R., T. Henn, P. G. Koehler, and C. L. Tucker. Microbial Insecticides. Vol. Vol. 1295. Cooperative Extension Service: University of Illinois at Urbana-Champaign, 1989.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 Ghazanchyan, N. L., M. H. Kinosyan, P. E. Tadevosyan, N. S. Khachaturyan, and E. G. Afrikian. “Brevibacillus Laterosporus as Perspective Source of New Bioinsecticides.” Annals of Agrarian Science 16, no. 4 (December 1, 2018): 413–15..
- ↑ 13.0 13.1 13.2 13.3 Oliveira, Edmar Justo de, Leon Rabinovitch, Rose Gomes Monnerat, Liana Konovaloff Jannotti Passos, and Viviane Zahner. “Molecular Characterization of Brevibacillus Laterosporus and Its Potential Use in Biological Control.” Applied and Environmental Microbiology 70, no. 11 (November 2004): 6657–64.
- ↑ Cai, Jian, Dianyu Chen, Dong Chen, Xianghu Huang, Changling Li, Huiling Liu, Mengjie Li, Guanbao Li, and Yulei Zhang. “Complete Genome Sequence of Brevibacillus Laterosporus Bl-Zj, an Algicidal Bacterium Isolated from Soil.” Microbiology Resource Announcements 8, no. 30 (July 25, 2019): e00408-19.
- ↑ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 15.11 Prasanna, Lakshmi, Vincent G. H. Eijsink, Richard Meadow, and Sigrid Gåseidnes. “A Novel Strain of Brevibacillus Laterosporus Produces Chitinases That Contribute to Its Biocontrol Potential.” Applied Microbiology and Biotechnology 97, no. 4 (February 2013): 1601–11.
- ↑ 16.0 16.1 Orlova, Margarita V., Tatyana A. Smirnova, Lyudmila A. Ganushkina, Victoria Y. Yacubovich, and Roudolf R. Azizbekyan. “Insecticidal Activity of Bacillus Laterosporus.” Applied and Environmental Microbiology 64, no. 7 (July 1998): 2723–25.
- ↑ 17.0 17.1 17.2 17.3 17.4 Ruiu, Luca. “Brevibacillus Laterosporus, a Pathogen of Invertebrates and a Broad-Spectrum Antimicrobial Species.” Insects 4, no. 3 (September 5, 2013): 476–92.
- ↑ Laubach CA (1916) Spore-bearing bacteria in water. J Bacteriol 1:505-512
- ↑ Favret, Montgomery E., and Allan A. Yousten. “Insecticidal Activity of Bacillus Laterosporus.” Journal of Invertebrate Pathology 45, no. 2 (March 1, 1985): 195–203.
- ↑ Elieh-Ali-Komi, Daniel, and Michael R Hamblin. “Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials.” International Journal of Advanced Research 4, no. 3 (March 2016): 411–27.
- ↑ Kramer, Karl J, and Subbaratnam Muthukrishnan. “Insect Chitinases: Molecular Biology and Potential Use as Biopesticides.” Insect Biochemistry and Molecular Biology 27, no. 11 (November 1, 1997): 887–900.
- ↑ Soberón, M., Gill, S.S. & Bravo, A. “Signaling versus punching hole: How do Bacillus thuringiensis toxins kill insect midgut cells?” Cellular and Molecular Life Sciences 66, 1337–1349 (January 2009)
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2023, Kenyon College
