Chlorobium ferrooxidans
Classification
Domain: Bacteria
Phylum: Chlorobi
Class: Chlorobia
Order: Chlorobiales
Family: Chlorobiaceae
Genus: Chlorobium
Species: ferrooxidans (2)
NCBI link to find]
Species
|
NCBI: Taxonomy |
Chlorobium ferrooxidans
Description and Significance
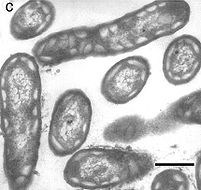
This green phototrophic bacterium is short, rod-shaped, approximately 0.5 x 1.0-1.5 μm in size, with rounded ends. The organism is nonmotile, gram negative, and nonsporeforming. Chlorobium ferrooxidans is strictly anaerobic. Originally isolated from shallow freshwater ditches, the phototrophic bacterium (strain KoFox) has only been isolated as a coculture with a strain identified as a member of the ε-subclass of the proteobacteria closely related to Geospirillum arsenophilum (KoFum). When grown in coculture, Chlorobium ferrooxidans oxidizes ferrous iron to ferric iron with stoichiometric formation of cell mass from carbon dioxide. This bacterium is important due to the fact that it is a novel green phototroph, related to other species of Chlorobium yet unique in regards to the oxidation of ferrous iron to ferric iron. This process by bacteria is a relatively novel phenomenon that has only been observed with phototrophic purple sulfur or non-sulfur bacteria (3, 4, 5). This observation in green phototrophic bacteria may indicate phototrophic ferrous iron oxidation was a widespread metabolic capacity in an early phase of evolution (1).
Genome Structure
The Chlorobium-like partner (KoFox) in the coculture is genetically related to Chlorobium, Prosthecochloris, and Pelodictyon, however no relationship was found to any strain for which rRNA sequence data currently are available (1). Overall 16S rRNA sequence similarity values of 91.4 - 96.7% indicate that the strain represents a separate line of descent within a Pelodictyon/Prosthechloris cluster (see figure 2). According to the NCBI Genome project website (2), the genome of Chlorobium ferrooxidans is 2.53896 Mbp in length, contains 2158 proteins and 47 RNAs.
Cell Structure, Metabolism and Life Cycle
The phototrophic bacterium (KoFox) is thought to obtain trace nutrients from the Geospirillium-like partner species (KoFum) while living together in a coculture. Selective enrichment from freshwater ditches and ponds has been achieved with optimal pH ranging from 6.3 - 7.1, and a temperature optimum between 22 - 25 C. Bacteriochlorophyll c is present in the organism and makes this bacterium strictly phototrophic with an affinity for dim light excluding light of 740 nm in wavelength. This strian (KoFox) differs from all Chlorobium strains in its lack of sulfide oxidation. Instead KoFox oxidizes ferrous iron to ferric iron hydroxides and uses a combination of iron and sunlight to reduce carbon dioxide for making and assimilating organic compounds. Hydrogen and acetate have been shown to increase the cell yield of the strain KoFox in the presence of ferrous iron. Chlorobium ferrooxidans has also been shown to have the capacity to use hydrogen as a sole electron source. Additionally, no assimilation of organic substances other than acetate have been found in C. ferrooxidans. The Oxidation of ferrous iron to ferric iron by KoFox in the presence of KoFum is coupled to biomass formation from Carbon dioxide according to the following equation:
17 FeCO3 + 29 H2O --> 17 FE(OH)3 + <C4H7O3> + 13 CO2 (1).
Ecology and Significance
The bacterium discussed here lives in a co-culture which seems to suggest a symbiosis of some sort. The description according to Heising et. al, the paper responsible for the initial description of this organism, explained that the iron oxidizing strain (KoFox) was unable to be separated from the Geospirillum-like chemoheterotrophic partner strian (KoFum). Compared to other green phototrophs (i.e. Chloroflexus aurantiacus, Chlorobium limicola, Pelodictyon phaeoclathratiforme,) strain KoFox behaves atypically in not utilizing sulfide as a sole electron source (1). The oxidation of ferrous iron by phototrophic bacteria is a relatively novel phenomenon that has typically only been observed with phototrophic purple sulfur or non-sulfur bacteria (3,4,5). Oxidation of ferrous iron by Phototrophic bacteria has had implications in our understanding of early earth evolution. Banded iron formations (BIF's) (FIGURE 4) are Precambrian sedimentary deposits that generally consist of alternating layers of iron minerals and silica. BIFs have proved particularly useful to studies of the early earth due to their intimate coupling with oxygen concentration (9), however have remained enigmatic and debated among the scientific community regarding the mechanism surrounding their formation. Biological mechanisms can potentially account for the precipitation of iron out of solution in a variety of environments, ranging from an anoxygenic photic zone to an oxygenated sub-photic zone (9). However, the production of oxygen at depths anticipated for BIF deposition remains debated. Importantly, concentrations of nutrients (P) and trace metals (V, Mn, Co, Zn, and Mo) found in iron-rich BIF bands can easily support microbe populations capable of precipitating Hamersley-scale BIFs – even during periods of maximum iron precipitation (10). The question of when oxygen evolved on the planet and whether it was present in sufficient concentrations to be responsible for the deposition of these BIF's is still under debate. The finding of Chlorobium ferrooxidans and the observation of ferrous iron oxidation by a green anoxygenic phototrophic bacteria may fuel further speculations on the question of how banded iron formations originated. Green phototrophs represent a separate group within the Bacteria domain, this may be an indication that phototrophic ferrous iron oxidation was a widespread metabolic capacity in an early phase of evolution during the Precambrian era, as well as add to the argument that oxygen was not necessary for the formation of these early geologic formations (1,6).
References
(1) Heising, S., Richter, L., Ludwig, W., and Schink, B. 1999. Chlorobium ferrooxidans sp. nov., a phototrophic green sulfur bacterium that oxidizes ferrous iron in coculture with a “Geospirillum” sp. strain. Arch Microbiol. 172:116-124.]
(2) NCBI Genome Project [1]
(3) Widdel, F. Schnell, S., Heising, S., Ehrenreich, A., Assmus, B., Schink, B. 1993. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature. 362:834-836.
(4) Ehrenreich, A. and Widdel, F. 1994. Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl. Environ. Microbiol. 60:4517-4526.
(5) Heising S. and Schink, B. 1998. Phototrophic oxidation of ferrous iron by a Rhodmicrobium vannielii strain. Microbiology. 144:2260-2269.
(6) Kappler, A., Pasquero, C., Konhauser, K. O., and Newman, D. K. 2005. Deposition of banded iron formations by anoxygenic phototrophic Fe(II)-oxidizing bacteria. Geology. 33:865-868.
(7) Beatty, J.T.; Overmann, J.; Lince, M.T.; Mansket, A.K.; Lang, A.S.; Blankenship, R.E.; Van Dover, C.L.; Martinson, T.A.; Plumley, F.G. “ An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent”. PNAS June 28, 2005 vol. 102 no. 26 9306-9310
(8) www.britannica.com
(9) Harnmeijer, J.P. 2003. Banded iron-formation: A continuing enigma of geology. University of Washington. http://www.reference.com/go/http://earthweb.ess.washington.edu/~jelte/essays/BIFs.doc.
(10) Konhauser K., Hamada T., Raiswell R., Morris R., Ferris F., Southam G., Canfield D. 2002. Could bacteria have formed the Precambrian banded iron formations? Geology, 30, pp. 1079-1082.
Author
Page authored by Paul Giordano and Apram Ghuman, students of Prof. Jay Lennon at Michigan State University.