Chronic Wasting Disease (CWD): Pathogenesis and History of a Silent Killer
Introduction

By Max Stafford
Chronic Wasting Disease(CWD) is a contagious neurological disease that affects cervids that is caused by prions, with confirmed cases in 24 different states of the US and 2 providences of Canada, since its original discovery 63 years ago in mule deer herds in Colorado[1][2][3]. CWD has also had confirmed cases in Norway, Finland, Sweden, and South Korea in smaller numbers[4]. Cases have come from both free-range and farm raised cervids. CWD causes a degeneration of infected animals, resulting in emaciation, abnormal behavior, loss of bodily functions, and death. Chronic Wasting Disease belongs to the group of diseases known as Transmissible Spongiform Encephalopathies(TSEs)[5]. CWD is a terminal disease that was discovered over 50 years ago[2], and still there are many unknowns about this disease, which has developed a reputation of being one of the most deadly and mysterious diseases known to man that isn’t a household name.
History
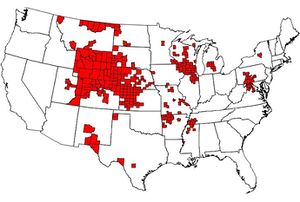
As of January 2020, there were 299 counties in 24 states with reported CWD in free-ranging cervids. This map is based on the best-available information from multiple sources, including state wildlife agencies and the United States Geological Survey(USGS). Provided by the CDC.(1)
The exact location and mode of CWD development is unknown, but the first observation of the condition occurred in 1967 during research being conducted upon mule deer herds in Colorado, and wasn’t confirmed as a TSE until 3 years later in 1970[3]. CWD was identified in captive facilities in both Colorado and Wyoming in mule deer, black-tailed deer, and elk soon after. In 1981, the first wild case was identified in Colorado in an elk, with mule deer cases following closely behind in 1985 in both Colorado and Wyoming. Endemic zones were established in both states, but CWD still spread. In the mid-1990’s to 2000, CWD spread to Saskatchewan, Canada via captive herds and then to wild cervids. Oklahoma and Oklahoma followed shortly after. By 2001, CWD was present in white-tailed deer, specifically in South Dakota wild herds and in a captive herd located in Nebraska. Soon, CWD spread to Minnesota, Wisconsin, New Mexico, Utah, Illinois, Kansas, Virginia, North Dakota, Iowa, and Pennsylvania due to the wide range and spread of white-tailed deer. Texas had its first confirmed case in 2012, Ohio followed with its first case in 2014 and Michigan right after in 2015. As of January 2020, there are confirmed cases of CWD in free-ranging deer, elk and/or moose in at least 24 states in the continental United States(1), as well as two provinces in Canada[1][2][6]. Other cases have been reported in other nations, such as Norway, Finland, Sweden, and South Korea[4][6].
CWD Entry and Pathogenesis
Chronic Wasting Disease belongs to the group of diseases known as Transmissible Spongiform Encephalopathies(TSEs)[5], better known as prion diseases. This is a group of rare degenerative brain disorders characterized by tiny holes that give the brain a "spongy" appearance. Other similar diseases include; scrapie, bovine spongiform encephalopathy(BSE) or “mad cow disease”, and transmissible mink encephalopathy, which are all found in domesticated animals[6]. There are a few rare human TSEs, such as Creutzfeldt-Jakob(CJD) and its variant v-CJD[7][6].
Transmission/Entry:
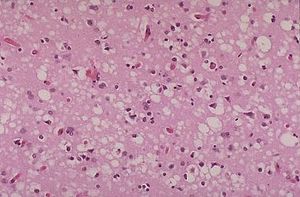
CWD proteins, or prions, spread between animals through the use of bodily fluids such as feces, saliva, blood, or urine through direct contact and indirect contact[8]. Indirect contact occurs through environmental contamination that comes through the common medium of soil, food, or even water[8]. So when CWD is introduced to a population, the prions spread quickly and can remain in an area for a long period of time, so other animals in the population can contract CWD from the environment, long after an infected host has passed through or died[8]. Transmission during utero can occur as well, but is extremely rare[5]. There is much more that needs to be done to fully understand how CWD is spread, due to the spread of cases and many variables that come into play for each population.
Pathogenesis:
CWD is a prion, which is an infectious protein that replicates by converting a normal cellular protein into copies of itself through conformational change[9]. So once the CWD enters the body of a host through either direct or indirect contact, it begins replicating through converting/conforming the normal cellular proteins of the host into copies of itself. Over time, these proteins begin to build up throughout the body and along the nerves, making its way to the brain, where the CWD begins forming the tiny holes that are stereotypical of TSEs [7][6]. At this point in time, CWD has been saturating and building up within the animal. The infected animal will begin to display symptoms as it may start to act unusually, such as excessive salivation, loss of appetite, progressive weight loss, increased thirst and urination, listlessness, teeth grinding, holding the head in a lowered position and drooping ears[5][6]. The animal may also become more docile as it will have lost all sense of the fight or flight instinct. Death follows soon after many of these symptoms, with the window of incubation being around 16 months, and around 2 years before the symptoms begin to show[6], which indicate the deer won’t last much longer after that.
CWD Structure
CWD is a protein, or Prion, that converts normal cell proteins into copies of itself[9]. Prions themselves differ from other infectious agents in that they are formed mostly of an abnormally folded prion protein and devoid of detectable nucleic acid created from PrPC[10]. PrPC is the protein that makes prions, and is found throughout the body. It is found in healthy organisms, but when PrP is found in CWD and other TSEs, the PrPC has a different structure that makes it resistant to proteases, enzymes that the body naturally uses to break down proteins. These proteins are known as PrPSc, and they are relatively polydisperse and defined poorly unlike PrPC. And because of this, the precise structure of prions is unknown. They are formed by combining PrPC, polyadenylic acid, and lipids in a protein misfolding cyclic amplification (PMCA) reaction, which is why it is devoid of detectable nucleic acid. The prion itself consists mostly of PrPSc, a β-sheet-rich conformer[4], which is how the CWD forces a normal PrPC cell to take on its form[9], thus replicating itself. Prions although structurally not really known, do possess a well defined loop that connects the second alpha helix with the beta sheet[11]. This structure in CWD is unique in comparison to other TSE, as it is usually flexibly disordered in humans and bovines. This loop is believed to create a species barrier for this prion disease[11], as there are multiple strains of CWD which infect different cervids.
PrPC:
PrPC is a normal protein found on the membranes of cells located in healthy organisms. PrPC has 209 amino acids (in humans), one disulfide bond, and a molecular mass of 35–36 kDa with an alpha-helical structure. The normal protein cannot be separated by centrifugation techniques. Its function is a complex issue that continues to be investigated, as it is not fully understood yet. For example; PrPC binds copper (II) ions with high affinity, which is presumed to relate to PrP’s structure or function. PrPC itself is readily digested by proteinase K and can be liberated from the cell surface in vitro by the enzyme phosphoinositide phospholipase C. PrP has been reported to play important roles in cell-cell adhesion and intracellular signaling in vivo, and may therefore be involved in cell-cell communication in the brain.[12]
PrPSc:
The infectious isoform of PrP, known as PrPSc, or simply the prion, is able to convert normal PrPC proteins into the infectious isoform by forcing them to change their conformation. This changes the way the protein is interconnected. PrPSc always causes prion disease, which is highly lethal with a 100% chance of death. The amount of time between infection and death varies from case to case. Although the exact 3D structure of PrPSc is unknown, it is known that it contains a higher proportion of β-sheet structure instead of the normal α-helix structure. Aggregations of these different isoforms form highly structured amyloid fibers, which accumulate to form plaques. The end of each fiber acts as a template onto which free protein molecules may attach, allowing the fiber to grow. So under most circumstances, only PrP molecules with an identical amino acid sequence to the PrPSc are incorporated into the growing fiber, meaning some is safe from the PrPSc. Cross-species transmission is also possible, but is incredibly rare.[13]
Host Response and Treatment
Signs and Symptoms:
Most cases of CWD occur in mature cervids; the youngest cervid to exhibit clinical symptoms of the CWD was 15 months old[14]. The disease is progressive and always fatal. Symptoms can take a long time to show up, as CWD has a 16 month incubation period[11], and then takes up to two years to even show symptoms, but this is different case by case[6]. The first signs shown tend to be difficulties in movement. This includes stumbling about, listing, and lowering of head. The movement itself can be repetitive in the way the cervid walks or moves. Weight loss is another consistent clinical sign of CWD, which consistently gets worse over time as the cervid eats less and less. Behavioral changes also occur in the majority of cases, with decreased interactions with other animals, tremors, and nervousness. Although the loss of fear in humans and confusion is common. Excessive salivation and grinding of the teeth has also been observed. Most deer show increased drinking and urination; the increased drinking and salivation may contribute to the spread of the disease[5]. These symptoms have given rise to CWD being given the nickname of ‘zombie deer disease’ by media outlets such as ABC News or CNN.
Diagnosis:
Diagnosis is usually based off of post-mortem examination and testing, although examination of the corpse is not definitive, as many cervids die early in the course of the disease, and may have other underlying conditions, as well as many of the conditions found are nonspecific. Upon microscopic examination, lesions of CWD in the central nervous system resemble those of other TSEs. Scientists also use immunohistochemistry to test brain, lymph, and neuroendocrine tissues for the abnormal prion protein of CWD. As of now, there is no reliable way to detect CWD in all live cervids, but in mule and white-tailed deer, tonsillar biopsy has been reliable[15]. Biopsies of rectal mucosa have been efficient in detecting CWD in mule and white-tailed deer as well as elk[16][17][18]. Detection efficiency in all methods can be affected by animal age, genotype, and stage of disease the cervid is in, which means they aren't always accurate.
Population Effects and Prevention:
Investigations of Chronic Wasting Disease effects on free‐ranging cervids suggest that the disease can cause long‐term population declines within the various herds of cervids, from deer to elk[14]. Death due to CWD has increased greatly over time as it has spread further to more and more herds around the world, from wild free-range cervids to captive herds[14]. And as we have observed this spread, and the increase of death via CWD, there are still many unknowns when it comes to the effects of CWD on cervid populations. Long-term effects of CWD on cervid populations and ecosystems remain unclear as the disease continues to spread and prevalence increases[14]. In captive herds, CWD might persist at high levels and lead to complete herd destruction in the absence of human culling, while game wardens and various environmental programs have done their best to slow the spread of CWD[19]. Other forms of prevention have been introduced, such as relaxed rules on deer hunting in an attempt to lower the population with CWD as well as collect more samples[19]. Harvest stations have also popped up all around in order to test for the disease, as well as keep count in the total of deer harvested by the Department of Natural Resources(DNR), as well as regulate the movement of harvested specimens across borders(States/Countries)[14]. Carcass incinerating services have also been introduced.
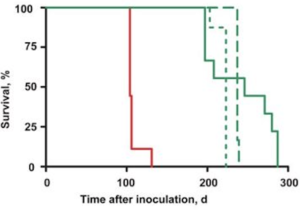
Treatment:
Currently there is no known treatment for Chronic Wasting Disease as it is a terminal disease. Once a cervid contracts the disease, it is only a matter of time before the cervid dies. Treatments are being developed currently using 2-aminothiazole to treat white-tailed deer CWD prions inoculated in transgenic mice(2). The 2-aminothiazole, or IND24 has been shown to be effective against elk CWD prions. When IND24 was introduced to the WTD CWD isolate infected mice, the survival of the mice was extended just like the elk CWD prion infected mice, yet they were considerably shorter than that of the elk CWD prion mice. Elk mice treated with IND24 daily survived 216 ± 6 days after inoculation, while the WTD mice survived 200 ± 6 after inoculation(2). This suggests that there are differences between the two strains. Different dosage sizes also affected the amount of time that the mice survived after inoculation. The treatment with IND24 itself didn’t alter the characteristics of PrPSc in the brains of the mice as well as the infectivity and drug susceptibility of CWD. And although IND24 extends the life of mice infected with different strains of CWD, it does not cure CWD.[20]
CWD and Humans:
CWD may be known as the “zombie deer disease” by various news outlets such ABC News and CNN, and as such only affects cervids, but CWD has zoonotic potential that people should be aware of. CWD has been able to infect squirrel monkeys and laboratory mice that carry genes shared with humans[8]. Through intracerebral inoculation; cattle, sheep, goats, mink, ferrets, and voles were also able to be infected with CWD[6]. In macaques, a type of monkey that is closer genetically to humans than any other animal infected before, CWD was transmitted to the monkey via infected meat they were fed[8]. This meat came from both infected deer and elk, with some from asymptomatic deer. CWD was also able to spread when infectious material was placed directly into their brains[8]. These transmissions occured in a lab, and there has been no confirmed cases of interspecies transmission of CWD to non-cervids, including carcass scavengers and cattle.The transmission between cervids and humans has never occured, and all studies show that potential for transmission to humans is low[3]. This is due to the low efficiency of CWD PrPSc and its ability to convert human PrPC to PrPSc[21]. When transgenic mice are expressing human genes, they become resistant to CWD infection as well[21]. And it is because of even the slightest possibility, humans have taken the precautions to avoid CWD crossing over to the human population. This is why there are so many preventative measures put forth, including avoiding the consumption of infected meat and proper disposal of infected dead cervids[5].
Conclusion
Chronic Wasting Disease is one of the most dangerous diseases out in the world today that hasn’t infected mankind. In the time since it was first discovered, CWD has spread from Colorado all the way around the globe in a little over 50 years, and yet many aspects about the disease are still a mystery to scientists. The terminal disease has the power to wipe out whole herds of cervids through turning a body's own proteins against itself. Research that has been conducted on CWD has found that CWD is a prion that is part of a group of diseases called Transmissible Spongiform Encephalopathies(TSEs), and its structures are largely made up of PrPSc composed of β-sheet structure. Even with this, much is still unknown about CWD’s structure as well as transmission and pathogenesis. All this has gone under the radar for mainstream media, other than little tidbits on ‘zombie deer disease’ that come up once in a while. This is all due to CWD not infecting humans yet and its low possibility of even doing that. But because CWD is such a deadly disease, we should be concerned. If CWD made the jump to humans, we would be left with no way to treat CWD. There is no cure for CWD, only a way of slowing its effects, and even that has only been in laboratory settings. This is why there has been such a push to prevent the spread of CWD and avoid the possibility that CWD jumps from cervid to humans, as well as protecting the natural resources that cervids provide. Should we be worried? No, but more awareness should be put out to help combat CWD in order to protect ourselves and the cervids that suffer from this horrible disease. This messed up protein, if left unchecked, may go down in history with its uncanny ability to provide a slow painful death by making the body turn against itself.
References
- ↑ 1.0 1.1 “Occurrence”. Centers for Disease Control and Prevention(CDC) 29 January 2020. Web. 14 April. 2020.
- ↑ 2.0 2.1 2.2 “History of Chronic Wasting Disease”. Texas A&M University Retrieved April 14, 2020
- ↑ 3.0 3.1 3.2 Bian, J., Napier, D., Khaychuck, V., Angers, R., Graham, C., & Telling, G. (2010). Cell-Based Quantification of Chronic Wasting Disease Prions. Emerging Infectious Diseases 10(6), 977-984. https://dx.doi.org/10.3201/eid1006.031082.,
- ↑ 4.0 4.1 4.2 Herbst, A., Velásquez, C. D., Triscott, E., Aiken, J. M., & McKenzie, D. (2017). Chronic Wasting Disease Prion Strain Emergence and Host Range Expansion. Emerging infectious diseases, 23(9), 1598–1600. https://doi.org/10.3201/eid2309.161474
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 “Chronic Wasting Disease FAQ”. CWD-INFO.org 14 April. 2020.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 The Center for Food Security & Public Health. October 2016. Retrieved 14 April. 2020.
- ↑ 7.0 7.1 “Transmissible Spongiform Encephalopathies Information Page”, National Institute of Neurological Disorders and Stroke(NIH) 27 March 2019. Web. 14 April. 2020.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 “Transmission” Centers for Disease Control and Prevention(CDC) 25 February 2019. Web. 14 April. 2020.
- ↑ 9.0 9.1 9.2 J., Napier, D., Khaychuck, V., Angers, R., Graham, C., & Telling, G. (2010). Cell-Based Quantification of Chronic Wasting Disease Prions. Journal of Virology, 84(16), 8322–8326. doi: 10.1128/jvi.00633-10
- ↑ A. L., Kelly, A. C., Green, M. L., Shelton, P., Novakofski, J., & Mateus-Pinilla, N. E. (2015). Prion protein gene sequence and chronic wasting disease susceptibility in white-tailed deer (Odocoileus virginianus). Prion, 449–462. https://doi.org/10.1080/19336896.2015.1115179
- ↑ 11.0 11.1 11.2 C. J. (2008). A prion disease of cervids: Chronic wasting disease. Veterinary Research, 39(4), 41. doi: 10.1051/vetres:2008018
- ↑ E, Solis GP, Schrock Y, Geiss C, Luncz L, Thomanetz V, Stuermer CA (March 2009). Weissmann C (ed.). Regulation of embryonic cell adhesion by the prion protein. PLOS Biology. 7 (3): e55. doi:10.1371/journal.pbio.1000055. PMC 2653553. PMID 19278297.
- ↑ TD, Sigurdson CJ (2016). Cross-species transmission of CWD prions. Prion. 10 (1): 83–91. doi:10.1080/19336896.2015.1118603. PMC 4981193. PMID 26809254.
- ↑ 14.0 14.1 14.2 14.3 14.4 "Chronic wasting disease of cervids: Current knowledge and future perspectives". Annual Review of Animal Biosciences. 3: 305–25. doi:10.1146/annurev-animal-022114-111001. PMID 25387112.
- ↑ Lisa L.; Conner, Mary M.; Baker, Thomas H.; Dreitz, Victoria J.; Burnham, Kenneth P.; Williams, Elizabeth S.; Hobbs, N. Thompson; Miller, Michael W. (2002). "Evaluation of Antemortem Sampling to Estimate Chronic Wasting Disease Prevalence in Free-Ranging Mule Deer". The Journal of Wildlife Management. 66 (3): 564. doi:10.2307/3803124. JSTOR 3803124
- ↑ Chris; Hoeting, Jennifer A.; Wolfe, Lisa L.; Galloway, Nathan L.; Antolin, Michael F.; Spraker, Terry R.; Miller, Michael W.; Hobbs, N. Thompson (2015). "Age and Repeated Biopsy Influence Antemortem PRPCWD Testing in Mule Deer (Odocoileus Hemionus) in Colorado, USA". Journal of Wildlife Diseases. 51 (4): 801–810. doi:10.7589/2014-12-284. ISSN 0090-3558. PMID 26251986
- ↑ Bruce V.; Schneider, David A.; O’Rourke, Katherine I.; Gidlewski, Thomas; McLane, James; Allen, Robert W.; McIsaac, Alex A.; Mitchell, Gordon B.; Keane, Delwyn P. (September 2012). "Diagnostic accuracy of rectal mucosa biopsy testing for chronic wasting disease within white-tailed deer ( Odocoileus virginianus ) herds in North America: Effects of age, sex, polymorphism at PRNP codon 96, and disease progression". Journal of Veterinary Diagnostic Investigation. 24(5): 878–887. doi:10.1177/1040638712453582. ISSN 1040-6387. PMID 22914819
- ↑ Terry R.; VerCauteren, Kurt C.; Gidlewski, Thomas; Schneider, David A.; Munger, Randy; Balachandran, Aru; O'Rourke, Katherine I. (2009). "Antemortem Detection of PrP CWD in Preclinical, Ranch-Raised Rocky Mountain Elk ( Cevvus Elaphus Nelsoni ) by Biopsy of the Rectal Mucosa". Journal of Veterinary Diagnostic Investigation. 21 (1): 15–24. doi:10.1177/104063870902100103. ISSN 1040-6387. PMID 19139496
- ↑ 19.0 19.1 "Chronic wasting disease - What to expect if your animals may be infected". Canadian Food Inspection Agency. 1 April 2019. Retrieved 20 April 2020.
- ↑ D., Giles, K., Oehler, A., Bhardwaj, S., DeArmond, S. J., & Prusiner, S. B. (2015). Use of a 2-aminothiazole to Treat Chronic Wasting Disease in Transgenic Mice. The Journal of infectious diseases, 212 Suppl 1(Suppl 1), S17–S25. https://doi.org/10.1093/infdis/jiu656
- ↑ 21.0 21.1 S. E., Bartelt-Hunt, S. L., & Bartz, J. C. (2012). Occurrence, transmission, and zoonotic potential of chronic wasting disease. Emerging infectious diseases,18(3), 369–376. https://doi.org/10.3201/eid1803.110685
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2020, Kenyon College.
