Clostridium botulinum: An overview and the dangers of neurotoxicity and Botulism
By Emily Buckwalter
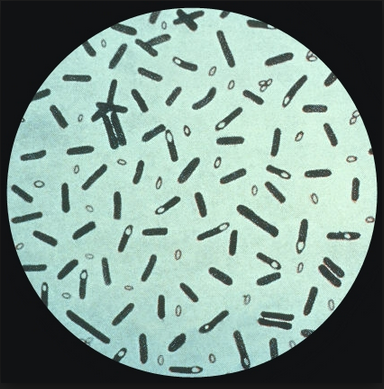
Clostridium botulinum. Image courtesy of Center for Disease Control (CDC). https://phil.cdc.gov/details.aspx?pid=2107
What is Clostridium botulinum?
Clostridium botulinum is a large Gram positive, motile, spore-forming, rod-shaped bacteria that ranges from 4-6 μm by 0.9-1.2 μm in length and width respectively[1]. Clostridium botulinum is a member of the family Clostridiaceae and of the order Clostridiales, under the branch of bacterial organisms known as firmicutes. This bacterial species is commonly known for its ability to produce a dangerous neurotoxin, botulinum (BoNT)[2]. Even the smallest amount of exposure to this neurotoxin causes the onset of foodborne botulism, as it has been ranked one of the six highest risk threat agents for bioterrorism by the Centers for Disease Control and prevention (CDC)[3]. Botulism is a lethal and potentially fatal disease with sudden onset that ultimately results in extreme muscle weakness and paralysis[4]. There are other recognized types of botulism caused by Clostridium botulinum, such as infant and wound botulism, but foodborne botulism is most commonly associated with this disease[5].
Given that this bacteria produces a lethal neurotoxin, it is classified as a pathogenic bacteria. Clostridium botulinum has also been divided into four distinct phenotypes, groups I-IV, all of which produce one of seven (A-G) types of BoNT[6]. Group I phenotypes are proteolytic, meaning that they are able to break down protein, specifically by lysing native proteins, whereas Group II phenotypes are non-proteolytic[7]. Group I and II are specific to infecting humans as their host organism. Group III and IV strains are not as well defined in their capabilities because they are produced in either animal only or neither animal or human diseases[2]. The seven types of BoTN follow in a similar fashion, where only types A, B, E and F target human hosts and for that reason are present in both Groups I and II phenotypes[8].
Clostridium botulinum is composed of chromosomal DNA that is 3,886,916 base pairs long. The known genome of this bacterial species has been found to contain approximately 3,650 genes. However, the plasmid of C. botulinum that integrates into host DNA is around 16,344 base pairs long, encoding for a total of 19 genes. The genes encoding BoNT have been suggested to be located in a cluster on the main chromosomal DNA or on the plasmid, however further research needs to be conducted to pinpoint an exact location[6]. While neurotoxin production is one of the widely studied functions of C. botulinum, most of the bacterial genome is responsible for encoding for proteases, enzymes that are used for the metabolism of proteins. In a study conducted by Sebaihia et al. 2007, C. botulinum was found to have very little newly acquired DNA, which tells us that the genome is very stable but also that there is not an extremely long relationship between the host organism and the bacteria, further emphasizing the role of the produced neurotoxin BoNT. Additionally this suggests that C. botulinum is well-adapted to its environment and can sufficiently carry out its own protection and metabolism without gene transfer to select for more optimal pathways[9].
Clostridium botulinum is of great importance to the food industry because it is one of many bacterial species that is commonly associated with the canning of foods, such as vegetables, that stock supermarket shelves. C. botulinum is found just about everywhere in the form of spores, a stage in the reproductive cycle of spore-forming bacterial species[2]. These spores can easily become trapped in the packaging process in food production plants, and without proper heat and pressure to kill off these microbial spores, the bacteria will continue to grow and reproduce[10]. Clostridium botulinum are also well known to be found in the soil thanks largely in part to the excretion of bacterial spores from human and animal intestines[11]. Once these bacteria are in the soil, they can still pose a threat by infecting the roots of plants and ultimately crops that are consumed by humans and other animals. Clostridium botulinum is also found in high abundance in aquatic environments, specifically sediments of oceans and lakes[8]. Researchers have been able to distinguish between the different types of BoTN found in these locations, which makes it easy to identify which phenotype of C. botulinum exists in that environment. For example, type E has been found in high concentrations in the ocean, linking it to cases of botulism involving contaminated fish, whereas type A and B have been linked to foods associated with soil contamination[8].
Life Cycle and Metabolic Properties
Clostridium botulinum grows and reproduces via the process of endospore formation and germination[2]. Most bacteria experience vegetative growth, whereby the cell divides in a 1, 2, 4… fashion and continues to multiply and divide. However in the case of spore-forming bacteria, given specific stimuli typically from the environment, the bacteria can pause vegetation growth and start germination[10]. Since Clostridium botulinum is able to form spores, this not only provides the cell with a means of protection but it also promotes the production of BoTN during the process of germination[12].
It is also known that spores can be activated based on environmental signals such as specific pH concentrations or temperatures, which is important especially in the food industry for means of food preservation[8]. Clostridium botulinum grows best under acidic conditions, specifically around a pH of 5[1]. Each of the two major groups (Group I and Group II) that target humans as hosts have their own optimal temperature for growth at 35℃ and 28℃ respectively. However, it is interesting to note that the Group II phenotype of C. botulinum, with BoTN types B, E and F, is able to generate neurotoxin at 3-4℃, which is standard refrigeration temperature[8]. This is dangerous especially for food production and safety. Therefore, it is important that any materials associated with food processing are properly sterilized, using extreme heat and pressure[10].
By selecting a temperature that is well out of the growth temperature for Clostridium botulinum, along with pressure, this will effectively ensure that almost every microbe has been killed so that they cannot grow once packaged and stored with food. This is one of the reasons why home-canned food is related to many recent outbreaks of botulism. Home canned foods typically can not ensure the same level of safety as commercial canner’s who are using equipment that produces extreme heat and pressure to kill any spores of C. botulinum that may be contaminating the packaging of the product. Some spores are able to resist the cooking and boiling process of at home canning processes[8].
The danger surrounding Clostridium botulinum presents itself under temperatures ranging from 40℉ to 120℉, as well as in anaerobic and low pH environments such as in a can, where these heat resistant spores can then convert and mature into growing cells[13]. Previous research has found that each C. botulinum group demonstrates different levels of heat resistance, with Group I spores being the most heat resistant, Group II being the least heat resistant. The spores of Clostridium botulinum have been able to achieve heat resistance through the structure of the spore formed[9].
Clostridium botulinum is an obligate anaerobe, meaning that it does not function under the presence of oxygen, and therefore must reside in low oxygen environments to prevent the toxicity of oxygen to the cells[2]. In order to metabolize sugars to produce energy for the bacterial cell, Clostridium botulinum primarily will use the anaerobic process of fermentation to produce energy. However it has been identified that some species of C. botulinum are able to use both fermentation pathways, while still being equipped with a glycolysis pathway[9]. The fermentation pathway consists largely of a series of oxidation-reduction reactions that are coupled and require electron transport via specific carriers such as NADH[10]. C. botulinum has been found to ferment specific amino acids including: glycine, proline, phenylalanine and leucine, all of which are nonpolar amino acids[9]. However one of the largest means of energy production for the bacterial species comes from the breakdown of chitin, a polysaccharide. The bacteria accomplish this via five different encoded enzymes, but also with the help of proteases which are able to cleave some of the large polysaccharide into more manageable, smaller molecules for metabolic pathways. Chitin is most commonly used by this bacterial species due to its relative presence in the environment. Chitin is most commonly found in the exoskeletons of arthropods and insects as well as in the cell walls of fungi, both of which are prevalent in marine and soil environments where Clostridium botulinum live. Additionally, chitin can be a source of supplementary carbon and nitrogen for the bacteria[6].
Dangers of Clostridium botulinum
Botulism, while rare, is a serious illness that grasps control of the body’s nervous system causing the nerves to enter a state of paralysis and permanent damage. Common symptoms include: muscle weakness, blurry vision, difficulty swallowing, vomiting and drooping eyelids. Exposure to the neurotoxin BoNT can rapidly trigger a wide range of symptoms, however all of the symptoms are as a consequence of muscle paralysis caused by BoNT. According to the CDC, foodborne botulism symptoms will begin to show approximately 18 to 36 hours after consuming food that has been contaminated by spores of Clostridium botulinum that have been able to grow and develop[14]. Given that this sudden and often fatal disease progresses at such a rapid rate, it is important that BoNT production is understood so that rapid detection of toxins is possible along with the development of potential treatments.
BoNT is a 150 k-Da polypeptide chain that is composed of a heavy and light chain, both with different crucial functions. The heavy chain is responsible for binding to nerve cells in the body and aiding in their entry into the nerve terminals. The light chain acts as a protease enzyme, specifically a zinc metalloprotease, that cleaves N-ethylmaleimide to produce sensor activating protein receptors, also known as SNARE proteins[15]. These SNARE proteins are able to block the other molecules in the synaptic cleft, most importantly the release of acetylcholine into the neuromuscular junction, which in turn causes the muscles to turn flaccid and enter a state of paralysis[16]. The speed in which BoNT displays neurotoxicity to a human host, for example, can be determined by an enzymatic step made by a protease of C. botulinum. The Group I phenotype of Clostridium botulinum is known to produce a specific proteolytic enzyme that cleaves BoNT to remove a small section, about 9 to 11 amino acids, between the heavy and light chains, leaving behind a disulfide bond and noncovalent interactions. This is considered an important proteolytic step that is rate-determining to kick-start the activation process that increases BoNT’s activity[17]. The Group II phenotype on the other hand has BoNT molecules with significantly lower activity, mainly due to the non proteolytic nature of this phenotype of Clostridium botulinum. This phenotype will have to rely on proteases from the host that C. botulinum infects, which it will gather upon entering the bloodstream and finding nerve cells to attack[18].
One of the most crucial steps in determining whether or not the neurotoxin BoNT is present after infection by Clostridium botulinum is detection. By detecting the presence of the toxin, treatment plans can then be made in hopes of preventing a fatal outcome for the human host. One method commonly used to detect BoNT is a “mouse bioassay” test which is able to determine what type A-G of the BoTN toxin is present using monoclonal antibodies and a blood sample from the organism. Next, an enzyme-linked immunosorbent assay (ELISA) is used to detect the presence of the toxin. ELISA is a plate-based assay technique that links an antibody to a reporter enzyme and is able to detect and quantify soluble substances such as the toxin BoNT[19]. From here, a quantitative PCR will be able to detect the toxin genes in the organism of interest, confirming that the host has been exposed to mature, toxin producing Clostridium botulinum[20].
Discovery and Historical Outbreaks
Historically, the relationship between food and paralytic disease was not recognized in many cultures, rather food was seen as a vital and sometimes scarce resource to the survival of many populations. Clostridium botulinum has been thought to be present since ancient times and throughout most of the 18th and 19th centuries based on recorded dietary taboos of certain populations. Examples of these cultural preparations and storage of foods that would promote the growth and development of C. botulinum include: poorly dried Herring in the Baltic, trout packed to ferment in baskets in Scandinavia and the storage of hams in brine in France[21]. While these methods promoted food preservation in times of uncertainty for those who needed to stretch their food rations, this also promoted the growth of parasitic bacteria and caused major outbreaks that at the time were unknown by researchers.
One of the largest and earliest outbreaks of what was assumed to be Clostridium botulinum, was in the end of the 18th century in the Württemberg region of Germany. There were a total of 6 deaths and thirteen people who were affected in total, of what was assumed to be atropine intoxication. However it was discovered in 1802, shortly after the incident, that the beloved famous dish, blood sausage, was to blame. This meat was cold-smoked meaning that the spores of C. botulinum were able to grow after not being properly killed off before consumption because the temperature reached was not high enough to degrade the proteins of the bacterial species and cause cell death. Originally the “sausage poisoning” was assumed to be due to the presence of prussic acid, however it wasn't until 1895 when Clostridium botulinum was discovered. In December of 1895 in the small Belgian village of Ellezelles, an overwhelming outbreak of close to 40 cases consumed the small village, suspected to be from pickled and smoked ham. Emile Pierre Marie Van Ermengem, of the University of Ghent, was credited with the discovery of Bacillus botulinus, which was later named Clostridium botulinum[21]. By means of thorough examination of the food and bodies of the deceased, he found that the victim's spleen as well as the ham itself had been filled with traces of anaerobic microbes. This discovery was vital to food production and preservation moving forward to prevent outbreaks of fatal disease and sparked further research into pathogenic bacterial species.
Another major outbreak was in the United States in the 1920s when a severe outbreak of botulism killed 18 people and was reported across multiple states including Michigan, Ohio and New York. This outbreak had been traced back to black olives that were canned and packed in California and had been damaged during the cross country shipping process, allowing temperature and pH changes to provide an environment for C. botulinum spores to thrive and grow[22]. This sparked the attention of the U.S. canning industry to pay close attention to safety measures to ensure proper heat and pressure in the sterilization of their products to prevent growth of parasitic bacteria and other microbes, and most importantly disease[23].
Treatments
Given the nature of these outbreaks, researchers have extensively studied Clostridium botulinum, and have developed treatments for those who are infected with this disease. Once a person has been diagnosed with botulism, typically after confirmation via a blood sample detecting the presence of BoNT, they will be admitted to the hospital. Antitoxin therapy is the main route of treatment for adults specifically infected with BoNT[4]. The drug equine antitoxin is administered to the patient either intravenously or through an intramuscular injection. Once administered, the drug is designed to display inhibitory effects on BoNT neurotoxins that have yet to bind to nerve cells, which in turn decreases the effects of muscle paralysis and opens the synaptic cleft to acetylcholine release. However this is not a one dose type treatment. There have been cases of botulism reported that last for a few months and require months to years of treatment to deplete symptoms of this disease[6]. Some specific antitoxin treatments include: GlaxoSmithKline trivalent Types ABE, NP-018 (heptavalent) Types A to G, and BabyBIG®, Botulism Immune Globulin Intravenous (Human) (BIG-IV) for children under the age of one. A vaccine was designed to protect uninfected individuals against botulism (types A-E), however it was discontinued in 2011 after reports of toxic shock resulted after administering the vaccine[4]. While great lengths have been made to combat botulism and there is only a 5-10% fatality rate once administered treatment, further research is required to target BoNT to dampen its effects on the nervous system[6].
Current Research and Clinical Applications
Based on research that has been conducted, the genes of Clostridium botulinum that encode the BoNT have been limited to only exist in that bacterial species thus far, until a 2018 study disproved that notion. Dong et al. 2018 discovered the presence of genes that closely mimic BoNT, named BoNT/En, in a strain of Enterococcus faecium which was isolated and sequenced from a sample of cow feces. While this seems unproblematic, E. faecium is a microbe that comprises part of the human gut microbiome[10]. Dong et al. 2018 found that BoNT/En was not toxic to humans or mice alone, however in conjunction with the transport subunit of BoNT that allows for passage across the nerve cell membrane, muscle paralysis was observed in a mouse leg. This sparks major concern to researchers because if these genes are able to be transferred from one species on a plasmid for example to a neighboring species that inhabits the human gut, BoNT related infection rates could skyrocket. Dong et al. claims that their research proves this concept to be highly unlikely in the human microbiome given that BoNT/En genes are most similar to insect metagenomes, but justifies the legitimacy of this concern[24].
Clostridium botulinum, while toxic and deadly to most organisms, has been adapted to use in clinical settings, most commonly as one of the primary ingredients in BOTOX. Botox injections work by weakening or paralyzing certain muscles or by blocking certain nerves, however the neurotoxin is at such a weak concentration that it is not toxic to the human body[25]. Clostridium botulinum has also been adapted as a treatment therapy for certain muscular conditions such as dystonia. Dystonia is a movement disorder in which muscles contract involuntarily resulting in repetitive or twisting movements. In the form of a localized injection, the botulinum neurotoxin is injected into muscles where it relaxes the muscles and reduces excessive muscle contractions without completely stopping muscle function. This is achieved by utilizing BoNT’s ability to control acetylcholine release from the synaptic cleft of nerve cells, to slow the release of acetylcholine and normalize muscle contractions and neuron signaling[26].
Conclusion
Clostridium botulinum has been widely studied and is known to predominantly arise from contaminated food sources that alot spores of the bacteria to develop and produce the harmful neurotoxin BoNT. However, further research is still needed to enhance the treatments options and their effectiveness against botulism. Additionally, future research would be beneficial to investigate the gene transfer capabilities of this bacterial species to ensure that this bacteria presents no greater threat to the human microbiome. Gaining a more comprehensive understanding of the BoNT neurotoxins and the mechanisms for their synthesis within C. botulinum can help generate sufficient treatment and protection.
References
- ↑ 1.0 1.1 Mckenzie, S. (2019, February 26). Clostridium botulinum life cycle. News. Retrieved April 15, 2022
- ↑ 2.0 2.1 2.2 2.3 2.4 Wikimedia Foundation. (2022, March 18). Clostridium botulinum. Wikipedia. Retrieved April 14, 2022
- ↑ Arnon SS, Schechter R, Inglesby TV, et al. Botulinum toxin as a biological weapon: Medical and public health management. JAMA. 2001;285:1059–1070.
- ↑ 4.0 4.1 4.2 Wikimedia Foundation. (2022, February 24). Botulism. Wikipedia. Retrieved April 14, 2022, from
- ↑ Schneider KR, Silverberg R, Chang A, Goodrich Schneider RM (9 January 2015). "Preventing Foodborne Illness: Clostridium botulinum". edis.ifas.ufl.edu. University of Florida IFAS Extension. Retrieved 7 February 2017.
- ↑ 6.0 6.1 6.2 6.3 6.4 Peck MW, Stringer SC, Carter AT (April 2011). "Clostridium botulinum in the post-genomic era". Food Microbiology. 28 (2): 183–91. doi:10.1016/j.fm.2010.03.005.
- ↑ Weigand Michael R., Pena-Gonzalez Angela, Shirey Timothy B., Broeker Robin G., Ishaq Maliha K., Konstantinidis Konstantinos T., Raphael Brian H., & Schaffner D. W. (2015). Implications of Genome-Based Discrimination between Clostridium botulinum Group I and Clostridium sporogenes Strains for Bacterial Taxonomy. Applied and Environmental Microbiology, 81(16), 5420–5429.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Center for Food Safety and Applied Nutrition. (n.d.). Bam Chapter 17: Clostridium botulinum. U.S. Food and Drug Administration. Retrieved April 15, 2022
- ↑ 9.0 9.1 9.2 9.3 Sebaihia, M., Peck, M., Minton, N., Thomson, N., Holden, M., Mitchell, W., Carter, A., Bentley, S., Mason, D., Crossman, L., Paul, C., Ivens, A., Wells-Bennik, M., Davis, I., Cerdeno-Tarraga, A., Churcher, C., Quail, M., Chillingworth, T., Feltwell, T., Fraser, A., Goodhead, I., Hance, Z., Jagels, K., Larke, N., Maddison, M., Moule, S., Mungall, K., Norbertczak, H., Rabbinowitsch, E., Sanders, M., Simmonds, M., White, B., Whithead, S., and Parkhill, J. 2007.
- ↑ 10.0 10.1 10.2 10.3 10.4 Slonczewski, Joan L., John W. Foster, and Erik Foster. Microbiology: An Evolving Science 5E. W. W. Norton & Company, 2020.
- ↑ Zhorzholiani, E., Chakvetadze, N., Katsitadze, G., & Imnadze, P. (2017). Spread of Clostridium botulinum in the soils of Georgia. Online Journal of Public Health Informatics, 9(1), e160.
- ↑ Webb, M., Stringer, S., Le Marc, Y., Baranyi, J., and Peck, M. 2011. Does proximity to neighbors affect germination of spores of non-proteolytic Clostridium botulinum? Food Microbiology: 32, 104-109
- ↑ Canning vegetables to prevent botulism. Canning Vegetables to Prevent Botulism | College of Agriculture, Forestry and Life Sciences | Clemson University, South Carolina. (n.d.). Retrieved April 15, 2022
- ↑ Centers for Disease Control and Prevention. (2021, June 1). About botulism. Centers for Disease Control and Prevention. Retrieved April 18, 2022
- ↑ Bandyopadhyay S, Clark AW, DasGupta BR, Sathyamoorthy V. 1987. Role of the heavy and light chains of botulinum neurotoxin in neuromuscular paralysis. J Biol Chem 262:2660–2663.
- ↑ Fredrick, C. M., Lin, G., & Johnson, E. A. (2017). Regulation of Botulinum Neurotoxin Synthesis and Toxin Complex Formation by Arginine and Glucose in Clostridium botulinum ATCC 3502. Applied and environmental microbiology, 83(13), e00642-17.
- ↑ Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006 Apr 28;312(5773):592-6.
- ↑ Dekleva ML, Dasgupta BR. 1990. Purification and characterization of a protease from Clostridium botulinum type A that nicks single-chain type A botulinum neurotoxin into the di-chain form. J Bacteriol 172:2498–2503.
- ↑ Overview of Elisa. Thermo Fisher Scientific - US. (n.d.). Retrieved April 18, 2022
- ↑ Satterfield BA, Stewart AF, Lew CS, Pickett DO, Cohen MN, Moore EA, et al. (January 2010). "A quadruplex real-time PCR assay for rapid detection and differentiation of the Clostridium botulinum toxin genes A, B, E and F". Journal of Medical Microbiology. 59 (Pt 1): 55–64.
- ↑ 21.0 21.1 Erbguth, F. J. (2004). Historical notes on botulism,clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Movement Disorders, 19(S8).
- ↑ Zeide, A. (2018, August 3). The Botulism Outbreak That Gave Rise to America’s Food Safety System. Retrieved April 18, 2022
- ↑ Henderson, R. D. R. by E. (2020, September 11). The use of microbes in food. News. Retrieved April 14, 2022
- ↑ Mansfield, M. J., Zhang, S., Lebreton, F., Miyashita, S.-I., Zhang, J., Schwartzman, J. A., Tao, L., Masuyer, G., Martínez-Carranza, M., Stenmark, P., Gilmore, M. S., Dong, M., & Doxey, A. C. (2018). Identification and characterization of a botulinum neurotoxin–like toxin in a commensal strain of enterococcus faecium. Toxicon, 156.
- ↑ [ https://medlineplus.gov/botox.html U.S. National Library of Medicine. (2021, December 14). Botox | botulinum toxin | botox injections. MedlinePlus. Retrieved April 18, 2022]
- ↑ Botulinum neurotoxin injections. Dystonia Medical Research Foundation. (n.d.). Retrieved April 18, 2022
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College