Clostridium difficile-associated disease

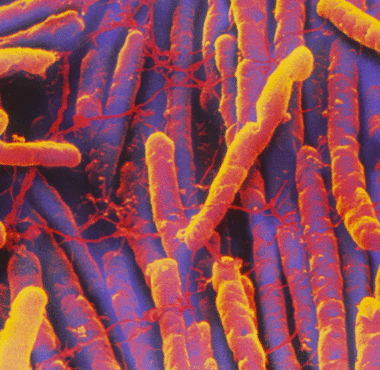
Etiology/Bacteriology
Taxonomy
| Domain = Bacteria | Phylum = Firmicutes | Class = Clostridia | Order = Clostridiales | Family = Clostridiaceae | Genus = Clostridium | species = Clostridium difficile
Description
Clostridium difficile is a Gram-positive, spore-forming rod (bacillus) that is an obligate anaerobe. It can be found in soil, water, feces, and the human gut. Infections of Clostridium difficile cause pseudomembranous colitis, toxic megacolon, perforations of the colon, sepsis, and, on occasion, death. C. difficile is a normal inhabitant of the gut microbial community of about 1-3% of adults. The pathogenic form of C. difficile is transferred via the fecal-oral route as well as through spore dispersal. Clostridium difficile-associated disease (CDAD) was initially reported approximately 30 years ago. The CDC first recorded infections from a hyper-virulent strain in 2000 along with a marked increase in the number of CDAD infections. C. difficile causes disease by producing the toxins TcdA and TcdB that function to disrupt protein synthesis within the host cell. The toxins are responsible for producing symptoms such as watery diarrhea, fever, loss of appetite, nausea, and severe abdominal pain [1]. Although C. difficile only causes about 20% of antibiotic associated colitis, standard treatments fail in about 25% of CDAD cases. Patients treated promptly typically recover. However, CDAD is notorious for recurrence after initial antibiotic treatment. 33% of patients with an infection will have a recurrence with 64% of those being within 30 days of the initial infection [2]. Complications typically develop in about 11% of patients in the first recurrence. This likely promotes the growth of antibiotic resistant strains that are able to perform horizontal gene transfer between recurrences. In about 20% of patients the infection will resolve itself in 2-3 of discontinuing the inciting antibiotic. Most infections that persist are treated with a 10-14 day course of antibiotics like metronidazole, vancomycin, and rehydration therapy. In more serious cases fecal transplants and surgery can be performed. [3] Patients at risk for developing CDAD include those taking antibiotics (especially broad spectrum), those taking proton pump inhibitors, GI manipulation or surgery, long term stays in hospital or clinical settings, immunocompromising conditions, and old age. The best practices for preventing infection include judicious administration of antibiotics, quarantine, hand hygiene, and the use of EPA-registered disinfectants with a sporicide (especially hypochlorite based disinfectants).
Pathogenesis
Transmission
Clostridium difficile are shed in feces, and therefore these bacteria can be transmitted via the fecal-oral route. The spores can survive on almost any surface for months to years which makes the pathogen very difficult to get rid of once established. The spores are resistant to many extreme environments, including high temperatures, ultraviolet light, harsh chemicals, and antibiotics. Health care settings, including patients and workers, are often the reservoirs for C. difficile spores. Community-acquired infections are thought to be transmitted through soil, water, pets, meats, and vegetables.
Infectious dose, incubation, and colonization
Since C. difficile is an opportunistic pathogen, the infectious dose and incubation period is unknown and widely debated. Exposure to broad spectrum antibiotics prior to infection is crucial to the pathogenesis, as C. difficile has a difficult time colonizing on its own, but it can be found as part of the normal gut microbiota of approximately 2-3% of the population. Once a patient has taken broad-spectrum antibiotics, C. difficile takes advantage of the lack of commensal bacteria in order to colonize the large intestine.
Epidemiology
Frequency
CDAD is most often a nosocomial (hospital-acquired) infection that causes an estimated 3 million cases of diarrhea and colitis per year. Some reports state that 28% of patients who were hospitalized tested positive for C. difficile. Its incidence in hospitals has risen from 30-40 per 100,000 in the 1990s to 84 per 100,000 in 2005 [4], and despite a decrease in other nosocomial infections from 2000-2009, the number of patients with CDAD discharge diagnosis more than doubled from approximately 139,000 to 336,600. In addition to this, the number of primary CDAD diagnoses more than tripled from 33,000 to 111,000. CDAD can be community-acquired, however the incidence of this is much lower than the hospital-acquired infections. The CDC’s Emerging Infections Program associated approximately 94% of CDAD diagnoses with receiving health care. Outside of the United States, the incidence of CDAD has also increased. For example, in one region of Quebec, its incidence quadrupled in 2003 to 92.2 per 100,000 populations.
Morbidity and Mortality
Though most patients with C. difficile can recover without specific therapy, symptoms may be particularly debilitating and drawn out. The elderly are much more susceptible to severe infection, and the mortality rate in this demographic is estimated to be as high as 25%. The disease’s mortality and morbidity seems to have increased in severity in the last decade. The CDC has reported that enteritis deaths more than doubled from 1997 to 2007 in the United States, increasing to 17,000 from about 7,000, and C difficile was associated with 14,500 of these deaths, up from 2,700 in 1999 [5]. A particularly virulent strain has been traced to several outbreaks in North America, known as the NAP1/027 strain. It shows increased production of toxins A and B, antibiotic resistance, and the production of a binary toxin whose role is not yet clear, but is thought to increase the virulence of the A and B toxins.
Virulence factors
C. difficile expresses two toxins, toxin A (TcdA) and toxin B (TcdB), which are two of the largest bacterial toxins known (review). They are part of the Large Clostridial Toxin family, in which the toxins glucosylate small GTPases in the cytosol of targeted cells. Both toxins disrupt the actin cytoskeleton of fibroblasts and prevent cells from being able to regulate actin polymerization. Toxin A produces a florid inflammatory response, while B has no enterotoxin activity, but instead is a potent cytotoxin. In fact, toxin B is 10 times more potent than A in causing damage in colonic epithelial cells, which points to toxin B as the primary virulence factor. The primary virulence factor for this bacteria has been disputed. It was previously thought to be toxin A, but recent evidence points towards toxin B.
Damage Response Framework
The damage response framework is a theory that measures the interaction between the pathogen and the host. This theory is able to give an explanation as to why the same pathogen can cause different levels of damage depending on the host. The interaction between the two parties can result in damage to the host, benefit to the host, or can have no effect. These levels of damage can then lead to circumstances of symbiosis, colonization, commensalism, latency, and disease.
Clinical features
Colonization of the intestine by Clostridium difficile can occur without presenting any symptoms in the host, however, infection can cause symptoms ranging from trivial diarrhea to serious manifestations of disease. General symptoms of CDAD include watery diarrhea, fever, loss of appetite, nausea, and abdominal pain/tenderness, but grossly bloody stools are unusual. Signs of more advanced disease include pseudomembranous colitis, toxic megacolon (also known as colonic distention), perforations of the colon, sepsis, and sometimes death.
One of the most well recognized manifestations of the disease, pseudomembranous colitis, was first described in 1893 and is the formation of lesions within the colon that are made of a pseudomembrane of immune cells, mucus, and necrotic tissue [4]. For 20% of patients with advanced CDAD: diarrhea and fluid loss are minimal, and instead, abdominal distention and bowel obstruction can lead to misdiagnosis. For other patients, signs of systemic toxicity and systemic inflammatory syndrome, including leukocytosis, rising serum lactate levels, hypotension, acute renal failure, and respiratory distress, lead to poor prognosis and high mortality rates. Fulminant colitis can lead to the need for a total colectomy, but even with this procedure, the average mortality rate at this stage of the disease is 67%, and the progression from initial symptoms to this stage can occur in as little as hours to as much as weeks.
Diagnosis
Prior to testing for Clostridium difficile, there are three predominate indications for the presumption of a C. difficile infection (CDI). Patients are most susceptible if they have received antibiotics within the last 8-12 weeks; patients are over the age of 64; patients have produced 3 or more diarrheal stools within 24 hours. The most widely accepted method used to test for CDI is a toxigenic culture involving the incubation of a fecal culture and subsequent immunoassay to test for the presence of C. difficile toxin A [6,7]. PCR is becoming a more popular method to identify toxins A and B because of its specificity, sensitivity, and rapid results. However, physicians warn that false positives are easily obtained from asymptomatic individuals. For this reason, physicians and researchers direct only to test diarrheal stool, especially from individuals who identify with any of the three preliminary traits listed above [6].
The application of a two-step technique involving an enzyme immunoassay (EIA) and a confirmatory step to detect the presence of C. difficile toxins in stool samples has also proven effective in clinical laboratories. The EIA is used to detect glutamate dehydrogenase (GDH); positive samples are then subject to toxigenic culturing. However, this method still requires further confirmation to determine the relative sensitivity and consistency among GDH test kits ([6,7].
In comparison to the EIA technique, the tissue cytotoxin assay shows higher specificity for toxin detection, but is a more complex and time intensive procedure. Tissue cytotoxin assays are considered inefficient in clinical settings [6].
Repeat testing from a single stool sample is not encouraged due to the increased risk for false-positive results [6,7]. Additionally, retesting a recently recovered patient may produce misleading results since C. difficile likely remains colonized for a period of time after CDI symptoms subside [6].
Treatment
Non-severe cases
For non-severe cases, the first step in treatment is the cessation of the inciting antibiotic as soon as possible and only continuing concomitant antibiotics if they are prudent to the treatment of the initial infection. Oral metronidazole or vancomycin are typically administered. However, some literature points to vancomycin having increased efficacy over metronidazole [8].
Moderate to severe cases
Antibiotics:
For more severe cases, higher, more frequent doses of vancomycin (either oral or rectal) are administered. However, rectal doses have a higher risk of producing colonic perforations and should only be administered when oral preparations are impossible for the patient.
Fecal Transplantation:
Another method of treatment is the infusion of a purified stool substitute preparation from a healthy donor. Generally, fecal transplantation is used in cases where the patient has had multiple recurrent infections and both metronidazole and vancomycin fail. It is reasonably successful in repopulating the gut with commensal bacteria and combating antibiotic resistant CDAD [9].
Probiotics:
Antibiotics function to alter the intestinal flora in order to produce an unfavorable environment for C. difficile. Some Lactobacilli, in particular, S. boulardii, have been shown to suppress C. difficile growth in hamsters. Furthermore, temporarily populating the gut with particular Lactobacilli can lower the pH of the surrounding environment and secrete degradation enzymes like proteases that place stress on the C. difficile population. Finally, some strains of beneficial bacteria may have the ability to protect the intestinal barrier by interfering TcdA and TcdB binding to the host gut epithelium [10].
Immunomodulation:
New research suggests that the supplementation of laboratory-derived monoclonal antibodies may have the ability to inhibit C. difficile colonization by stimulating toll-like receptors and upregulating expression of dendritic cells and peripheral blood monocytes [11].
Surgery:
Some severely ill patients with CDAD may require surgical intervention as a result of toxic megacolon, colonic perforations, necrotizing colitis, or infections producing systemic inflammatory responses that could potentially lead to organ failure. In these cases, diseased portions of the gastrointestinal tract are removed.
Prevention
Prevention with good hygiene habits will go a long way to protect against opportunistic pathogens like C. difficile. The main focus with C. difficile specifically should be on more conscientious antibiotic use, as broad spectrum antibiotics make patients susceptible in the first place. Restricting antibiotic use should lower the incidence of the disease and hopefully slow its growing virulence. When dealing with CDAD patients in a hospital setting, isolation of the sick, proper hand washing techniques, protective gowns and gloves, and cleaning with sodium hypochlorite are all important and effective ways to prevent outbreaks in healthcare settings. Work on a vaccine has begun, which appears to be promising, but is still far from market availability.
Host Immune Response
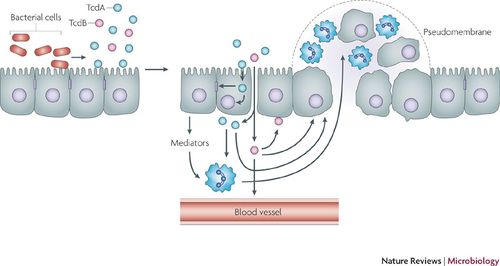
The pathophysiology of C. difficile is directly related to the host immune response to CDI. C. difficile colonizes in the lumen of the small intestine where it can lie dormant by means of sporulation, or it produces enterotoxins TcdA and TcdB eliciting an inflammatory immune response. Toxin A attracts polymorphonuclear cells (PMNs) or neutrophils to the site of infection via cytokines from epithelial host cells. This influx of leukocytes as a function of the immune response increases vascular permeability, allowing TcdA and TcdB to cross the mucosal membrane. TcdB degrades colonic epithelial cells. Accumulations of leukocytes form a pseudomembrane on the lining of the colon (pseudomembranous colitis).
Current research suggests that upon exposure to C. difficile, humans develop an adaptive immunity to TcdA and TcdB. It is estimated that approximately 60% of healthy adults exhibit IgG and IgA antibodies against C. difficile toxins. Studies suggest that adaptive immunity can begin in childhood from environmental exposure to C. difficile, and possibly non-toxigenic clostridial species [13,14]. Studies show that immunoglobulin A (IgA) antibody inhibits TcdA binding to epithelial cells in the colon [12]. Moreover, higher levels of IgA and IgG anti-toxins were found in asymptomatic patients, as well as those who displayed minor symptoms of CDAD [13,14].
References
1. Larson HE, Price AB, Honour P, Borriello SP: Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet 1978, 1: 1063-1066.
2. Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Godin D, Bourassa C: Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005 Jun 1; 40(11): 1591-7.
3. LaMont JT, Calderwood SB, et al.: Clostridium difficile in adults: Clinical manifestations and diagnosis. UpToDate June 2013, Available at: http://www.uptodate.com/contents/clostridium-difficile-in-adults-clinical-manifestations-and-diagnosis.
4. CDC. Vital Signs: Preventing Clostridium difficile Infections. MMWR Morb Mortal Wkly Rep. Mar 9 2012;61:157-62. [Medline].
5. Centers for Disease Control and Prevention (CDC). Deaths from gastroenteritis double. Available at http://www.cdc.gov/media/releases/2012/p0314_gastroenteritis.html.
6. Gould, Carolyn. CDC Commentary: Testing for Clostridium difficile Infection. Medscape. Aug 16, 2010.
7. Cohen S., Gerding D., Johnson S., Kelly C., Loo V., McDonald L., Pepin J., Wilcox M. (2010). Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America. Infection Control and Hospital Epidemiology 31(5):431-455.
8. Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 1986; 27: 1169.
9. Petrof EO, Gloor GB, Vanner SJ et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 2013 January; 1:3
10. Sartor RB, LaMont JT, Probiotics for gastrointestinal diseases. UpToDate June 2013, Available at: http://www.uptodate.com/contents/probiotics-for-gastrointestinal-diseases?source=see_link&anchor=H7#H7
11. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010; 362:197.
12. Kelly C. P., Pothoulakis C., Orellana J., Lamont J.T. (1992). Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A-receptor binding. Gastroenterology 102, 35-40. Pmid:1309359.
13. Kyne L., Warny M., Qamar A., Kelly C.P. (2000). Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 342, 390-397.
14. Viscidi R, Laughon BE, Yolken R, et al. Serum antibody response to toxins A and B of Clostridium
difficile. J Infect Dis 1983;148:93–100. [PubMed: 6886489]
Created by Laura Boucher, Marrett Hild, and Lillian Flannigan, students of Tyrrell Conway, PhD at the University of Oklahoma
Edited by Cassandra Long and Amber Hubbard, students of Tyrrell Conway, PhD at the University of Oklahoma
