Colwellia psychrerythraea
A Microbial Biorealm page on the genus Colwellia psychrerythraea
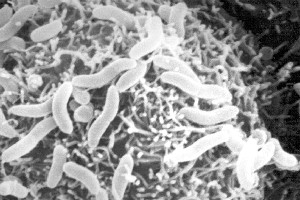
Classification
Higher order taxa
Domain: Bacteria; Phylum: Proteobacteria; Class: Gammaproteobacteria; Order: Alteromonadales; Family: Colwelliaceae; Colwellia
Genus
Colwellia psychrerythraea
Strain: 34H / ATCC BAA-681
|
NCBI: Taxonomy [2] |
Description and significance
Colwellia psychrerythraea is considered an obligate psychrophile, Gram-negative bacteria, and appears rod-shaped and red in pigment. This flagella-containing organism can be found in continually cold marine environments including Arctic and Antarctic sea ice. Strain 34H, in particular, was isolated from Arctic marine sediments. It has a growth temperature range of -1°C to 10°C. Optimal growth appears at 8°C, with maximum cell yield occurring at the subzero temperature of -1°C. Cells are able to swim in temperatures as low as -10°C. Growth can occur under deep sea pressures as well.
Sequence completion of this genome has revealed Colwellia's significant role in bioremediation, carbon and nutrient cycling, production of secondary metabolites, and cold-adapted enzymes. C. psychrerythraea is considered a model organism for the study of life in permanently cold environments, specifically bacterial adaptations. These adaptations include production of extracellular polymeric material for purposes of biofilm formation and cryoprotection, as well as enzymes with the ability to breakdown high-molecular-weight organic compounds. Particularly unique to this organism is the production of cold-active enzymes which show distinct heat instability and optimal activity occurring at low temperatures. These features make Colwellia species important in carbon and nutrient cycling in the cold marine environments in which they inhabit. From contaminated sediments to ice formations, observation of this organism can possibly give insight into earlier Earth environments as well as those on other planets and moons.
Genome analysis discloses C. psychrerythraea's flexible, yet complex means of acquiring carbon and nutrients, as well as generating energy, confirming this organism's evolutionary success and importance in environmental cycles.
Genome structure
The complete genome of Colwellia psychrerythraea 34H has been sequenced and shown to be 5,373,180 nucleotides in length.[1] The genome consists of one circular chromosome, of which 85% is predicted to be coding DNA. This DNA codes for 117 RNAs and 4910 proteins, including nine rRNA operons, 88 tRNAs and one structural RNA.[2] The DNA also contains 38% guanine-cytosine base pairing. [1]
Comparative genome analysis proposes that the psychrophilic behavior stems from a set of synergistic modifications in the overall genome content and amino acid composition, rather than a specific collection of genes responsible for such cold adaption. [2]
Genome completion has also revealed the existence of two filamentous phage genomes in the circular chromosome. Divergence locations of the two phage genomes suggest the possibility of a former recombination event with a single circular phage genome. Because of close proximity to integrases and transposases, which are involved in mobile insertion sequences, it is suggested the phage genomes may be part of a larger mobile genetic element. [2]
Cell structure and metabolism
Extra-cellular Compounds
This rod-shaped bacteria is enclosed by two cell membranes and contains flagella for motility. Important adaptations to its survival in such cold environments include production of extracellular polysaccharides which function in protection against cold-damage, known as cryoprotection. The genome has revealed a substantial amount of coding for an extracellular subfamily of σ-70 transcription factors, which among its many roles, can regulate extracellular polymeric synthesis. Genome analysis has also shown a large amount of coding for glycosyl transferases, which are suggested to produce such extracellular polysaccharides. [2] These extracellular polymeric substances (EPS) have been shown to increase extracellular enzymatic activity and stability in C. psychrerythraea. This stabilizing effect, which can potentially benefit several microbial generations, may allow for ever-increasing extreme environments to be tolerated. [7]
Genome analysis of C. psychrerythraea has also revealed a large amount of extracellular enzymes which may play a role in the metabolism of dissolved organic carbon at low temperatures. Of these enzymes, which function in peptide degradation, over half are expected to be located outside of the cytoplasm. This considerable amount of coding for extracellular enzymes is among the highest percentage in any completed genome. This suggests the importance of the ability to obtain organic carbon from the environment.[2]
Membrane Fluidity
Other cell features characteristic to cold adaption involve cell membrane fluidity and coding sequences for polyunsaturated fatty acid synthesis as well as a fatty acid cis/trans isomerase, which both aide in increasing cell membrane fluidity. [2]
Carbon and Nitrogen Reserves
Genomic investigations also show C. psychrerythraea produces polyhydroxyalkanoate (PHA) compounds, which function in intracellular carbon and energy storage. Means of nitrogen storage have also been identified in the production cyanophycin-like compounds, which serve as nitrogen reserves. Production of both of these compounds insures important intracellular carbon and nitrogen reserves when cold environments may set limitations on such uptake. [2]
Energy Metabolism and Electron Transport
Genome analysis discloses C. psychrerythraea's flexible, yet complex means of acquiring carbon and nutrients, as well as generating energy. The genome has revealed enzymes for many metabolic pathways including glycolysis, the citric acid cycle including the glyoxylate shunt, b-oxidation of fatty acids, pentose phosphate pathway, as well as fermentative reactions. This organism is also able to metabolize an array of carbon sources, from simple sugars to high-molecular-weight compounds. Extensive coding for peptide degradation also reveals the importance of carbon and nitrogen for energy metabolism. Purines can even be used a nitrogen source if required. Genomic studies have shown an enhanced role in sulfur metabolism as well, allowing for the uptake of sulfur for energy production and growth. This feature is especially useful in sedimentary environments, as well as in marine snow, where sulfur compounds can exist. [2]
C. psychrerythraea is considered a facultative anaerobe and can use oxygen as it's electron acceptor in the electron transport system. It also has anaerobic capabilities and can use alternative electron transport chains if necessary. This capability makes this organism particularly advantageous over others that cannot survive in anaerobic conditions. [2]
Unusual Capabilities
Upon genome analysis, genes coding for DNA metabolism, motility, and protein synthesis were found, which are similar to other Proteobacteria. However, some unusual genes were discovered including five cold-shock proteins. Four of these were identified to be in the cytoplasm, and the fifth has a unique protein structure of three transmembrane regions. [2]
An especially uncommon capability of this organism is the synthesis and utilization of co-enzyme F420. This co-enzyme was first found in methanogens where it is important in methanogenesis; however, it has been discovered in very few other organisms. Clues to the function of it in C. psychrerythraea can possibly come from examining the bacterial organism Rhodococcus which uses the co-ezyme for polynitroaromatic compound degradation. This suggests that C. psychrerythraea may use it for aromatic compound metabolism, an important ability for obtaining carbon sources. [2]
Because C. psychrerythraea uses aerobic respiration for energy when possible, an important feature is the ability to handle reactive oxygen species. Genome analysis has shown this organism to have an enhanced role in this area with not only the standard iron or manganese superoxide dismutase (SOD), but also an alternative nickel-containing SOD. This is advantageous when iron levels in the environment are low. [2]
Ecology
C. psychrerythrea's unique cold adaptations such as the abilites to synthesize and breakdown high molecular weight compounds,as well as carbon metabolism, make this organism important in carbon and nutrient cycling in such cold marine environments. This organism's ecological role not only includes catabolism of complex substance for carbon and energy reserves, but also detoxification activities which may aide in bioremediation of cold habitats. For example, genome analysis has shown the presence of enzymes with capabilities to degrade the toxic substance pentachlorophenol. [2]
Coding sequences for the complete denitrification process to dinitrogen gas have been indentified. This suggests C. psychrerythrea may play an important role in nitrogen cycling in cold, sedimentary environments. [2]
Pathology
This organism is not known to be pathogenic.
Application to Biotechnology
C. psychrerythraea 34H, along with many other bacteria that produce polyhydroxyalkanoate (PHA) compounds, as intracellular carbon sources, are of industrial interest for their thermoplastic and elastomeric properties, as well as a source of biodegradable plastic. Similarly, production of cyanophycin-like compounds that serve as nitrogen reserves, also has industrial potential of replacing non-degradable plastics. [2]
Genome analysis has revealed several detoxification capabilities in this organism, including enzymes which degrade toxins such as pentachlorophenol which have the potential to be used for biotechnological applications. [2]
Current Research
1. As of May 2007, scientists at the Department of Biomedicine at the University of Bergen, has solved the crystal structure for the cold-adapted enzyme phenylalanine hydroxylase (CpPAH) found in C. psychrerythraea 34H. With the aide of that structure, they analyzed the catalytic properties of the enzyme, and found that the cold-adaptive properties appear to involve increased flexibility around the active-site of CpPAH as well as hydrogen bond disruption for the enzyme's cofactor BH4. CpPAH exhibited highest activity at 25°C, which is 15°C above the optimal temperature growth. Characteristic of cold-active enzymes, high catalytic efficiency of the enzyme was observed at 10°C. However, unlike most cold-adapted enzymes, the half-inactivation and denaturation, known as the Tm, was found to be only slightly lower when compared to this enzyme's activity in non-psychrophilic organisms. This knowledge, along with observance of the crystal structure, suggests that cold-adaption for CpPAH involves increased flexibility, which does not largely effect the enzyme's thermostability. [5]
2. Researchers in Toulouse, France analyzed TonB-dependent receptors (TBDRs), which are outer membrane proteins primarily recognized for the uptake of iron siderophore complexes in Gram-negative bacteria, and their role in Proetobacteria, as well as Bacteroidetes. Genome analysis has identified large representation of these TBDRs, in both phytopathogenic, and aquatic bacteria including C. psychrerythraea. Genomic analysis of both types of bacteria show the TBDRs include a membrane transporter, an amylosucrase which aides in sucrose hydrolysis, and a sucrose utilization regulator; these make up a newly identified carbohydrate utilization locus, named CUT locus. These specific TBDRs and CUT loci have been found to be different from TBDRs in human gut Bacteroids, which allow for glycan scavenging. This suggests a convergent evolution relationship between TBDRs in Proteobacteria and Bacteroidetes. [6]
3. Scientists at the University of Washington, which have performed extensive research on C. psychrerythraea 34H, have discovered a new temperature minimum for metabolic activity in this organism, which involves incorporation of leucine into proteins at the low temperature of -20°C. Effects of added extracellular polymeric substances (EPS), which can aide in attachment, were also analyzed and found to enhance rates of leucine incorporation in temperatures -1 to -20°C. Unexpectedly, low rates of intracellular incorporation of leucine into protein were observed in samples with added EPS, at the low temperatures of -80 and -196°C. Explanation of such mechanisms is unclear but may involve salt and organic polymeric substances. Further analysis of bacterial extracellular-intracellular relationships could aide in the understanding of activity at sub-freezing temperatures. [4]
References
|
1.NCBI: Colwellia psychrerythraea 34H Genome Project at TIGR [3] |
Edited by Jaclyn Gaede; student of Rachel Larsen and Kit Pogliano
