Courtney Dever and Jennifer Lopez
Classification
Kingdom: Bacteria
Phylum: Firmicutes
Class: Bacilli
Order: Bacillales
Family: Paenibacillaceae
Species
|
NCBI: Taxonomy |
Genus: Brevibacillus species: laterosporus [1]
Habitat Information
Two tablespoons of an unknown soil organism were collected at a depth of approximately 1” below the soil surface on January 29, 2016 at 11:53 am from latitude 30.280954, longitude -97.760251, and placed in a plastic Ziploc baggie at room temperature. Included is a picture of the exact location where the soil was taken at the time of retrieval.
Weather conditions on January 29, 2016 at 11:53 am were as follows [2]
Wind: S14
Visibility: 10
Weather: A few clouds
Sky conditions: FEW 200
Temperature:
• Air temperature: 73°F
• Dewpoint: 34
• 6 hour max: 73
• 6 hour min: 32
Relative humidity: 24%
Pressure:
• Altimeter (in): 30.06
• Sea level (mb): 1017.8
• Precipitation (in): 0
Weather conditions also included [3]
ET0 or PET: 0.10 in.
Temperature max: 76 °F
Temperature min: 36°F
RH min: 17%
Solar radiation: 17.55 MJm2
Rain: 0 in.
Wind 4 am: 0.12 mph
Wind 4 pm: 6.13 mph
Soil conditions included [4]
Map Unit Name: Travis soils and urban land, 1 to 8 percent slopes
Acres in AOI: 0.1
Percent of AOI: 100%
Description and Significance
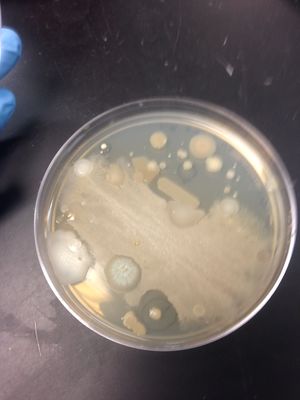
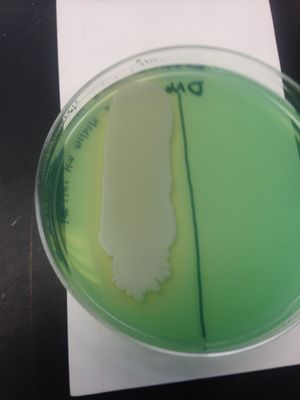
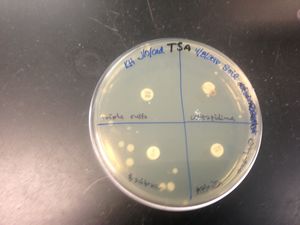
We chose colony #1 from our Master Patch Plate on E.coli because it showed a small clearing around the colony and hinted at antimicrobial properties.
Colony morphology:
-Size: small
-Margin: round and somewhat uneven
-Elevation:convex
-Surface: smooth
-Pigment produced: none
-Color: Whitish and opaque
Gram stain: positive
Endospore stain: positive
Capsule stain: negative
Brevibacillus laterosporus is a G+ rod (bacillus) that forms short chains of 2. It also forms endospores.
Brevibacillus laterosporus is a broad spectrum antimicrobial species due to its antimicrobial uses against phytopathogenic bacteria and fungi. [5]
Several antibiotics produced by Brevibacillus laterosporus include laterosporamine, laterosporin, tauramamide (active against pathogenic Enterococcus), and loloatin A (has cyanolytic activity). Brevibacillus laterosporus produces an antimicrobial substance that is effective against Gram-positive bacteria (Streptococcus, Staphylococcus aureus and Clostridium); Gram-negative bacteria, (Escherichia coli and Pseudomonas putrefaciens); and against Candida albicans. [6] It also exhibited strong antimicrobial characteristics against methicillin-resistant Staphylococcus aureus, vancomycin-resistant strains of Enterococcus faecalis and Lactobacillus plantarum, and other Gram-positive bacteria. In addition, Brevibacillin inhibits foodborne pathogenic and spoilage bacteria, such as Listeria monocytogenes, Bacillus cereus, and Alicyclobacillus acidoterrestris. [7] Also, Brevibacillus laterosporus has been shown to have antimycobacterial properties in a Brazilian study and has potential use against M. tuberculosis. [6] It has also been used as a probiotic in humans [8], mammals and birds. [5]
Genome Structure
Our organism was pipetted into well four of the electrophoresis gel shown in the attached photo. The genome of Brevibacillus laterosporus is small. It has one chromosome (5,106,578 bp) and 2 circular plasmids. [9] Generally, there is a single band of approximately 900 bp for all B. laterosporus samples that may serve as a molecular marker for this species. [10]
BLAST Search from DNA sequencing results: [11]
>CD-Forward_G10.ab1
GATGGAGCAACGCCGCGTGAACGATGAAGGCTTTC
GGGTCGTAAAGTTCTGTTGTTAGGGAAGAAACAGTGCTATTTAAATAAGATAGCACCTTGACGGTACCTAACGAGAAAGC
CACGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGTGGCAAGCGTTGTCCGGAATTATTGGGCGTAAAGCGCGCG
CAGGTGGCTATGTAAGTCTGATGTTAAAGCCCGAGGCTCAACCTCGGTTCGCATTGGAAACTGTGTAGCTTGAGTGCAGG
AGAGGAAAGTGGTATTCCACGTGTAGCGGTGAAATGCGTAGAGATGTGGAGGAACACCAGTGGCGAAGGCGACTTTCTGG
CCTGTAACTGACACTGAGGCGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGA
GTGCTAGGTGTTAGGGGTTTCAATACCCTTAGTGCCGCAGCTAACGCAATAAGCACTCCGCCTGGGGAGTACGCTCGCAA
GAGTGAAACTCAAAGGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGAAGAACCT
TACCAGGTCTTGACATCCCACTGACCGCTCTAGAGATAGAGCTTCCCTTCGGGGCAGTGGTGACAGGTGGTGCATGGTTG
TCGTCAGCTCGTGCCGTGANATGTCATA
>JL-Reverse_H10.ab1
ACCACCTGTCACCACTGCCCCGAAGGGAAGCTCTATCTCTAGAGCGGTCAGTGGGATGTCAAGACC
TGGTAAGGTTCTTCGCGTTGCTTCGAATTAAACCACATGCTCCACCGCTTGTGCGGGCCCCCGTCAATTCCTTTGAGTTT
CACTCTTGCGAGCGTACTCCCCAGGCGGAGTGCTTATTGCGTTAGCTGCGGCACTAAGGGTATTGAAACCCCTAACACCT
AGCACTCATCGTTTACGGCGTGGACTACCAGGGTATCTAATCCTGTTTGCTCCCCACGCTTTCGCGCCTCAGTGTCAGTT
ACAGGCCAGAAAGTCGCCTTCGCCACTGGTGTTCCTCCACATCTCTACGCATTTCACCGCTACACGTGGAATACCACTTT
CCTCTCCTGCACTCAAGCTACACAGTTTCCAATGCGAACCGAGGTTGAGCCTCGGGCTTTAACATCAGACTTACATAGCC
ACCTGCGCGCGCTTTACGCCCAATAATTCCGGACAACGCTTGCCACCTACGTATTACCGCGGCTGCTGGCACGTAGTTAG
CCGTGGCTTTCTCGTTAGGTACCGTCAAGGTGCTANCTTATTTAAATAGCACTGTTTCTTCCCTAACAACAGAACTTTAC
GACCCGAAAGCCTTCATCGTTCACGCGGCGTTGCTCCATCAGACTTTCGTCCATTGTGGAAAATTCCCTACTGCTGCCNC
CCGTA
Cell Structure, Metabolism and Life Cycle
An interesting feature of B. laterosporus is that it forms a canoe-shaped lamellar body on one side of its spores. It also produces cytoplasmic crystalline inclusions. [5]
From our biochemical tests, (namely, the Oxidase test), we were able to determine that our organism, B. laterosporus is an aerobe--it uses oxygen as the final electron acceptor in the electron transport chain system. From the Phenol Red broth tests, we know that B. laterosporus ferments glucose for energy.
B. laterosporus is able to produce polyketides, nonribosomal peptides, and toxins. [6]
Physiology and Pathogenesis
Enzymes made: gelatinase and DNase.
Other identifying characteristics: Gram + rod that produces endospores.
Brevibacillus laterosporus has been associated with human infections [12] and may cause septicemia. [13] It also may cause Endophthalmitis in humans, and produced severe panophthalmitis, corneal perforation, orbital cellulitis, and meningitis in rabbits. [14] Also, B. laterosporus is toxigenic in Chinese hamster ovary cells. [15]
Brevibacillus laterosporus also produces an extracellular protease which is inhibitive to mycelial growth of phytopathogens. [16] Some strains of B. laterosporus also produce chitinases that may play a major role in the degradation of the cell wall of fungi. [6]
B. laterosporus has the potential to be used as a biological control agent.
Brevibacillus laterosporus has been shown to have pesticidal uses against insects, nematodes and mollusks. [5] Also, toxicity towards beetles, larvae of the mosquitoes Culex quinquefasciatus and Aedes aegypti and the blackfly have also been reported. [11]
Biochemical Characteristics:
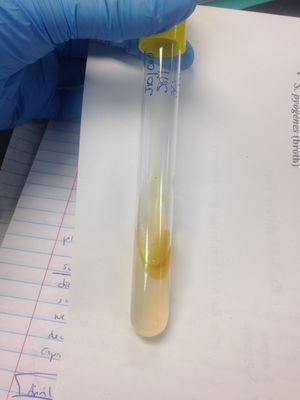
Phenol Red Broth:
•Glucose fermentation: (+) result; broth was yellow indicating glucose fermentation.
•Lactose fermentation: (-) result; broth was red—no change in broth.
•Sucrose fermentation: (-) result; broth was red—no change in broth.
problems encountered during procedure: no problems occurred during procedure.
Starch Hydrolysis: (-) result; clearing was not present on starch agar media; soil organism does not produce the α-amylase enzyme.
Casein Hydrolysis: (-) result; no clearing in skim milk agar; soil organism does not produce the casease enzyme.
Gelatin Hydrolysis: (+) result; solid broth turned to liquid; soil organism produces the gelatinase enzyme.
DNA Hydrolysis: (+) result; clearing in agar; soil organism produces the DNase enzyme.
Lipid Hydrolysis: (-) result; no clearing in Tributyrin agar; soil organism does not produce the Lipase enzyme.
Methyl Red: (-) result; broth is orange in color; soil organism does not use the mixed acid fermentation pathway, and does not produce mixed acid end-products.
Voges Proskauer (+) result; broth is red in color; soil organism utilizes the butylene glycol pathway, produces acetoin, and produces neutral end-products.
Citrate Test: (-) result, slant is green in color; soil organism does not utilize citrate as a carbon source.
SIM Tests:
•Motility (+) result; soil organism is motile.
•Indole (+) result; soil organism is able to produce indole. *indole result is questionable as top of media was pink
•Sulfur (-) result; soil organism cannot reduce sulfur to hydrogen sulfide.
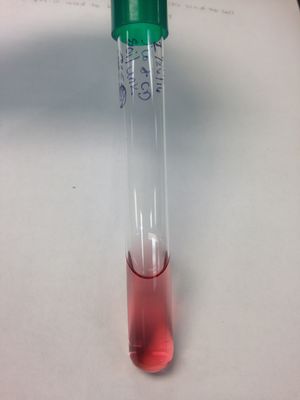
Nitrate Reduction Test: (-) result; soil organism is not able to reduce Nitrate to ammonia or molecular nitrogen; zinc was added the test tube turned red, showing a negative result for nitrate reduction.
Urea Hydrolysis: (-) result; broth is salmon in color; soil organism does not produce the urease enzyme. *result is questionable as the broth was not bright pink, nor was it orange or yellow
Triple Sugar Iron: (K/A) result; slant is red in color, butt is orange in color; glucose fermentation with acid production; peptones/proteins catabolized aerobically (in the slant) with alkaline products (reversion).
Decarboxylation:
•Arginine (-) result; broth is yellow; soil organism does not produce arginine decarboxylase and does not ferment.
•Lysine (-) result; broth is yellow; soil organism does not produce lysine decarboxylase.
•Ornithine (-)result; broth is yellow; soil organism does not produce ornithine decarboxylase.
Oxidase Test: (+) result; soil organism can produce Cytochrome c oxidase.
Eosin Methylene Blue Agar : (-) result; organism didn't grow b/c it is G+.
Hektoen Enteric Agar (HE): (-) result; organism didn't grow b/c it is G+.
MacConkey Agar (MAC): (-) result; organism didn't grow b/c it is G+.
Phenylalanine Deaminase: (-) result; organism doesn't produce Phenylalanine deaminase.
Catalase Test: (-) result; organism doesn't produce catalase.
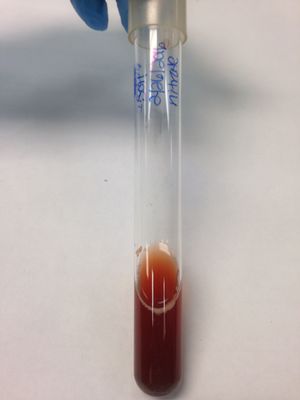
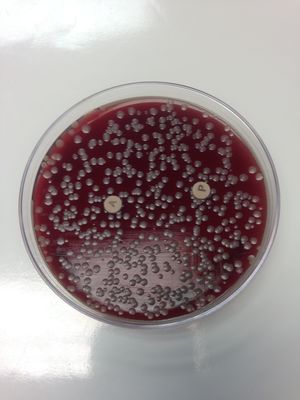
Blood Agar: (gamma) result; no clearing in the media; soil organism does not produce hemolysins.
Mannitol Salt Agar : (-) result; no growth and no fermentation.
Phenylethyl Alcohol Agar (PEA): (+) result; growth, but really slow growth.
Bile Esculin: (+) result; slant is dark brown in color; organism hydrolyzes esculin.
6.5% Salt Tolerance: (-) result; organism is not salt tolerant.
Bacitracin/Optochin: (-) result; organism produced no antibodies to A-disc or P-disc.
Antimicrobial Sensitivity/Kirby-Bauer Test:
•(-) to Triple Sulfa; organism is resistant to triple sulfa.
•(-) to Azlocillin; organism is resistant to azlocillin.
•(-) to Ceftazidime; organism is resistant to ceftazidime.
•(-) to Bacitracin; organism is resistant to bacitracin.
problems encountered during procedure: no problems occurred during procedure.
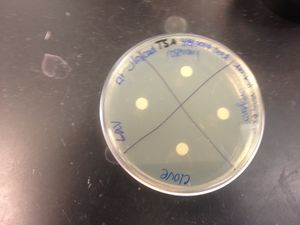
Disinfectant sensitivity:
•(-) to lavender; organism is resistant to lavender oil.
•(-) to clove; organism is resistant to clove oil.
•(-) to rosemary; organism is resistant to rosemary oil.
•(-) to oregano;organism is resistant to oregano oil.
problems encountered during procedure: no problems occurred during procedure.
References
National Institute of Health, US National Library of Medicine, National Center for Biotechnology Information's "Taxonomy Browser"
Retrieved from http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi [1]
National Oceanic and Atmospheric Administration's National Weather Service at http://w1.weather.gov/ [2]
Texas A & M Agrilife Extension at http://texaset.tamu.edu/ [3]
United States Department of Agriculture Natural Resources Conservation Service, Web Soil Survey
http://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm [4]
Ruiu, Luca "Brevibacillus laterosporus, a Pathogen of Invertebrates and a Broad-Spectrum Antimicrobial Species". Insects 2013 Sep, Volume 4(3), 476-492; doi:10.3390/insects4030476
Retrieved from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4553477/?tool=pmcentrez and
www.mdpi.com/2075-4450/4/3/476/pdf [5]
Hassi, M., S. El Guendouzi, A. Haggoud, S. David, S. Ibnsouda, A. Houari, and M. Iraqui1. "Antimycobacterial activity of a Brevibacillus laterosporus strain isolated from a Moroccan soil." Braz J Microbiol. Vol 43.4 (2012 Oct-Dec): 1516–1522.
Retrieved from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3769022/ [6]
Yang, X., E. Huang, C. Yuan, L. Zhang, and A. Yousef. "Isolation and Structural Elucidation of Brevibacillin, an Antimicrobial Lipopeptide from Brevibacillus laterosporus that Combats Drug-Resistant Gram-Positive Bacteria." Appl. Environ. Microbiol. vol. 82 no. 9 (May 2016): 2763-2772.
Retrieved from: http://aem.asm.org/content/82/9/2763.short [7]
Logan, N.A. "Bacillus and relatives in foodborne illness." Journal of Applied Microbiology, Vol 112 (2012): 417–429.
Retrieved from: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2672.2011.05204.x/full [8]
Djuvic, Marvin, Anja Poehlein, Andrea Thürmer, and Rolf Daniel. "Genome Sequence of Brevibacillus laterosporus LMG 15441, a Pathogen of Invertebrates." Journal of Bacteriology, 2011, Volume 193, No. 19, pg 5535-5536.
Retrieved from: http://jb.asm.org/content/193/19/5535.full [9]
Oliviera, Edmar Justo de, Leon Rabinovitch, Rose Gomes Monnerat, Lian Konovaloff Jannotti Passos and Viviane Zahner. "Molecular Characteristics of Brevibacillus laterosporus and Its Potential Use in Biological Control." Applied Environmental Microbiology, Nov 2004, Vol 70, No. 11, 6657-6664.
Retrieved from: http://aem.asm.org/content/70/11/6657.full [10]
National Institute of Health, US National Library of Medicine, National Center for Biotechnology Information's BLAST
Retrieved from: http://blast.ncbi.nlm.nih.gov/Blast.cgi [11]
Sanders, M.E., L. Morelli, and T.A. Tompkins. "Sporeformers as Human Probiotics: Bacillus, Sporolactobacillus, and Brevibacillus." Comprehensive Review in Food Science and Food Safety Vol 2 (2003): 101-110.
Retrieved from: http://onlinelibrary.wiley.com/doi/10.1111/j.1541-4337.2003.tb00017.x/epdf [12]
Saleh, R., M. Schorin. "Bacillus sp. sepsis associated with Hickman catheters in patients with neoplastic disease." Pediatric Infectious Disease Journal
Vol 6.9 (September 1987).
Retrieved from: http://journals.lww.com/pidj/abstract/1987/09000/bacillus_sp__sepsis_associated_with_hickman.13.aspx [13]
Khalid, T.; F. Juffali; R. Matossian. "Bacillus laterosporus Endophthalmitis" Arch Ophthalmol. Vol 95.12 (1977):2187-2189.
Retrieved from: http://archopht.jamanetwork.com/article.aspx?articleid=632440 [14]
Rowan, N., G. Caldow, C. Gemmell, and I. Hunter. "Production of Diarrheal Enterotoxins and Other Potential Virulence Factors by Veterinary Isolates of Bacillus Species Associated with Nongastrointestinal Infections." Applied and Environmental Microbiology Vol. 69.4 (Apr. 2003): p. 2372–2376.
Retrieved from: http://aem.asm.org/content/69/4/2372.full.pdf+html [15]
Zhang, Ying. "The antifungal protease from Brevibacillus laterosporus." Chinese Journal of Biological Control Vol 22.2 (2006):146-149.
Retrieved from http://europepmc.org/abstract/cba/619068 [16]
Author
Page authored by Courtney Dever and Jennifer Lopez, students of Prof. Kristine Hollingsworth at Austin Community College.