Crohn’s Disease Relating to the Gut Microbiota Specifically Faecalibacterium prausnitzii
By Kayla Arone
Background
General Crohn’s Disease Information
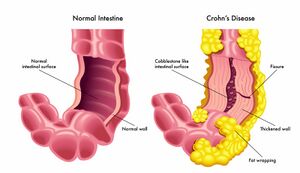
Crohn’s Disease (CD) is a form of inflammatory bowel disease (IBD) caused by the swelling of the tissues in the digestive tract. It can affect many segments or parts of the small or large intestine either continuous or non-continuously [1]. The symptoms can vary from mild to severe and can gradually increase. Common symptoms of Crohn’s Disease may include diarrhea, fever, fatigue, blood in stool, reduced appetite and weight loss, abdominal pain, and cramping, which have the ability to worsen overtime [2].
The direct cause of Crohn’s Disease remains unknown, although genetic predisposition plays a role as well as certain viruses or bacteria are believed to contribute to this disease [1]. There is a complex interplay between many factors throughout the gut that triggers this inflammatory response, leading to the disease's symptoms [1]. When the body's immune system fights off invading microorganisms an immune response that is not normal causes the immune system to not only fight off the bacterium but also cells in the digestive tract classifying Crohn’s as an autoimmune disease attacking the healthy bacteria as well [1]. The immune system attack does not turn off, and it stays active creating inflammation even when the infection goes away [1].
While there is no known cure for Crohn’s Disease, there are many therapies and clinical trials that can greatly manage symptoms. Medications are also extremely effective at improving these symptoms to prevent inflammation in the intestines [2]. For example, steroids, and anti-inflammatory drugs such as aminosalicylates which is a chemical related to aspirin are some treatment options [2]. Additionally, a new treatment approach includes tumor necrosis factor (TNF) inhibitors along with other injectable treatments for more severe cases targeting the gut microbiota which are still being explored [1].
The gut microbiota has been established as a big role in the development of Crohn's Disease and specific alterations to gut homeostasis and bacteria levels, are found in patients with CD. When looking for new treatment options, researchers have been turning to the gut microbiota as a source to target for potentially new and upcoming treatment options for improved outcomes and quality of life for individuals with this chronic condition.
Intestinal Microbiota
Colonization of the gastrointestinal tract of infants starts directly after birth and occurs within the birth canal and the mother's skin [3]. The type of diet that the mother uses can also affect the colonization pattern [4]. Other extenuating factors relating to health disparities such as country of origin, and birthplace may also be a factor in the development of an infant's gut microbiota [5]. Startup bacteria from birth regulate the expression of genes in host epithelial cells which harbors a likable environment for the bacteria further preventing the growth of other bacteria that may be introduced later [4]. Over time, this initial population of microbes diversifies and stabilizes in the gastrointestinal tract [4]. This colonization is responsible for and determines what the composition of the GI tract is like for adults as they get older [4].
The analysis of fecal flora is important in understanding the taxonomic identification of bacteria present in the gut microbiota [6]. Analyzing fecal bacteria relies on cultivating bacteria in various growth mediums which allows for researchers to identify the different types of bacteria present based on their growth characteristics. This method provides evidence supporting that the gut microbiota harbors a wide range of bacteria, with more anaerobic bacteria by 100 to 1000 times more versus aerobic bacteria [6]. The main taxonomic groups present were concluded to be the genera bacteroids, bifidobacterium, eubacterium, clostridium, peptococcus, peptostreptococcus, and ruminococcus which are predominant in human beings whereas aerobes (facultative anaerobes) such as escherichia, enterobacter, enterococcus, klebsiella, lactobacillus, proteus, etc are among the subdominant genera [6]. Each human has many hundreds of bacteria relating to this genre with a combination that is independent and distinct from any other individual [6]. The composition of each person's gut flora also can deviate under specific conditions such as antibiotic treatment, dietary interventions, and diarrheal illness, but for the most part, there is constancy [6]. All of these different bacteria encompass the gut microbiota and work to live in homeostasis with one another in order to create a healthy balanced gut.
Gut homeostasis is the state where host functions that control microbial growth are normal for a healthy and normally functioning individual [7]. There are a variety of innate immune cells that maintain in this gut homeostasis for example the promotion or suppression of T-cell differentiation and activation [2]. A diverse and balanced gut microbiota with a sufficient amount of beneficial bacteria such as F. prausnitzii is needed for keeping the gut lining healthy and preventing further inflammation [7]. When there is an imbalance and disruption to this gut homeostasis, as well as a decrease in beneficial bacteria it can lead to Crohn’s Disease [7].
Faecalibacterium prausnitzii
Genome Structure and General Information
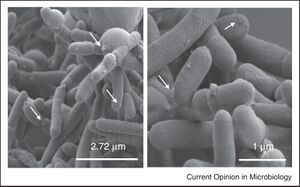
Faecalibacterium prausnitzii (F. prausnitzii) is a Gram-negative bacterium, non-spore-forming is extremely oxygen-sensitive [8]. This type of gut bacteria is one of the most abundant bacteria in the human gut, accounting for 5-15% of the total bacterial population in the gut [8]. It is very prevalent in human populations as it is detected in 85% of gut samples and F. prausnitzii is considered to be a very beneficial bacteria for the gut [8]. Levels of F. prausnitzii differ by age and gender as well, with lower levels in women than men. Levels of F. prausnitzii can be first detected around 6-7 months of age and the abundance rises at around 2-3 years old suggesting that there are different evolutionary stages of F. prausnitzii [8]. Decreased F. prausnitzii levels are observed in various forms of inflammatory bowel disease (IBD), Crohn’s disease (CD), ulcerative colitis (UC), and colorectal cancer (CRC) [8].
Although F. prausnitzii is a Gram-negative bacterium, it resembles characteristics of a Gram-positive bacteria such as those in Clostridium cluster IV [7]. Gram-negative bacteria usually have LPS found on their outer membrane, however, this is not present in F. prausnitzii suggesting that this is a bacterium that does not perfectly align with its classification. F. prausnitzii was initially classified as a member of the Fusobacterium genus of the Firmicutes phylum [7]. After a 16S rRNA sequencing, F. prausnitzii was found to be a new genus that is broken into two phylogroups and three clusters [7].
Many factors contribute to cultivating a healthy gut microbiota and the continued maintenance of a healthy diet, specifically polyphenols, which are micronutrients in order to have the fruition of this good bacterium in the gut and help regulate gut homeostasis [7].
Anti-inflammatory Properties and Butyrate Production

F. prausnitzii produces butyrate which is a short-chain fatty acid that nourishes the cells that line the colon and promotes anti-inflammatory effects [9]. The supernatant of F. prausnitzii can regulate T-cell differentiation. T-cells have been considered to be closely related to the occurrence and severity of intestinal inflammation [9]. They are part of the immune system and develop from stem cells in bone marrow. F. prausnitzii's job is to help produce energy to anti-inflammatory metabolites that are needed for intestinal health [9]. F. prausnitzii strains have a decreased ability to use polysaccharides which are found in the gut lumen such as xylan and soluble starches however are adapted well to the gut environment [9]. F. prausnitzii functions to ferment prebiotics to stimulate the production of good bacteria in the gut [9]. With this, F. prausnitzii can ferment glucose into formate, acetate, and most importantly butyrate, a necessity for a healthy gut.
Butyrate also plays a key role in the anti-inflammatory regulation of the gut microbiota [10]. There are many anti-inflammatory effects such as the inhibition of NF-κB activation in colonic epithelial cells [10]. When such a pathway is activated, there is an increase in pro-inflammatory cytokines including TNF-α and IL-1β in the immune system causing an immune response [10]. When there becomes an imbalance in the production of pro-inflammatory cytokines, and NF-κB is highly activated, it is linked to colon cancer and Crohn’s disease curating high levels of inflammation [10].
Although F. prausnitzii is a major butyrate producer in the gut, it is not limited to only butyrate also producing carbon dioxide, formate, D-lactate as well as consuming acetate [7].
F. prausnitzii was studied in greater detail to determine its anti-inflammatory effects and to understand the mechanism by which this occurs. In the supernatant of F. prausnitzii researchers found that this bacteria can help regulate T helper 17 cells (Th17) which are linked to inflammation along with regulatory T-cell differentiation by balancing secreting substances [10]. There was already previous research that hinted to the supernatant having anti-inflammatory effects, but it was not specifically clear. Mice with colitis (IBD) were treated with the supernatant as a result, they had improved weight loss, colon shortening, and tissue damage confirming the point that the supernatant reduced inflammation in the colon [10]. Furthermore, mice with colitis had more Th17 immune cells which are the cells linked to inflammation, and less T regulatory cells to regulate the inflammation. F. prausnitzii supernatant drastically reduced the Th17 cells and cultivated the amount of Treg cells [10].
Microbial Remediation of Crohn’s Disease
Intestinal Microbiota in Crohn’s Patients
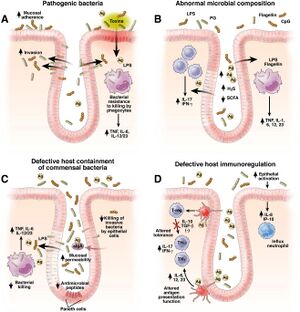
Patients with Crohn’s Disease contain an altered gut microbiota than those with a healthy functioning gut microbiota as outlined earlier. Their gut microbiome will have a smaller variety of communities of bacteria but with less of certain bacteria such as F. prausnitzii which is a health-promoting microorganism [4]. There is more harmful bacteria called Klebsiella pneumoniae which is an anaerobic bacterium that resides in the mouth. With Crohn's, as there is increased inflammation, there will be higher levels of oxygen in the gut microbiota [4]. Klebsiella overpowers the other bacteria that cannot handle the high levels of oxygen leading to more inflammation and more microbial imbalance which in turn worsens the symptoms [4]. In the UK, six different hospitals found that patients with CD had higher levels of antibodies against Klebsiella suggesting that patients with CD have had more exposure or been more affected by Klebsiella [11].
The composition of the mucosa-associated microbes in Crohn’s Disease patients provides insight into looking at commensal bacteria in the gut microbiota of Crohn’s disease patients [4]. The pathogenesis of Crohn's involves the activation of the mucosal immune system functioning to protect the mucus membrane against colonization and invasion of pathogenic bacteria as well as preventing the uptake of foreign antigens [4]. However, an imbalance in this system often triggered by intestinal microbiota can lead to inflammation. Two strategies called, the “candidate microorganisms strategy” and the “global description strategy” are researched to understand the role of microorganisms in Crohn’s Disease due to the reduced diversity of F. prausnitzii [4].
Further research showed a decrease in the F. prausnitzii levels associated with the risk of endoscopic recurrence and inflammation within six months post-surgery in CD patients [12]. Furthermore, probiotics such as Lactobacillus johnsonii La1 and Lactobacillus salivarius Ls33 demonstrated a potential reduction in inflammation preventing this recurrence and offering a therapeutic aid [12].
Probiotics
As inflammation is seen to decrease with the abundance of F. prausnitzii, the next step can be to figure out how to remediate the gut microbiota in order to begin to restore gut homeostasis. Probiotics are microorganisms that have an advantageous benefit when consumed or applied [13]. In relation to gut dysregulation, probiotics have a great potential therapeutic effect for patients with an imbalance in gut microbes. The main effect in relation to CD is to reintroduce the beneficial bacteria and especially increase the production of F. prausnitzii to produce anti-inflammatory metabolites in order to reduce symptoms [7].
One study analyzed and conducted a pan-genome analysis of 84 F. prausnitzii strains in order to determine which strains would be safe to use for probiotic treatment [7]. It was found that the majority of the strains were isolated from the feces of humans going with the finding that F. prausnitzii is a dominant bacteria in the intestinal tract of humans [7]. The core genome of F. prausnitzii as well as the pan-genome were compared [7]. With the greater number of strains, the genome size of the core genes decreased finding only 375 core genes that were identifiable [7]. Only 4.5% of the pan-genome was left unchanged while 95.5% was different between the strains showing that the pan-genome had higher genomic variability [7]. The genetic factors of 84 F. prausnitzii strains were different and contributed to the adaptation of F. prausnitzii strains in the intestine. Therefore, it is important to notice that different strains may have different properties and effects on different patients. Most of the genes found for the essential core function of F. prausnitzii were found in the accessory genome, and therefore not present in all strains [7]. With further analysis, it was concluded the the complete fatty acid metabolism pathways in F. prausnitzii showed a potential role in gut nutrient processing [7]. Finally, the probiotic potential risk index (PPRI) and probiotic potential risk score (PPRS) were calculated for each of the F. prausnitzii strains studied [7]. Figure 1 points to the 84 F. prausnitzii strains that are classified into three different groups being a low-risk probiotic, medium-risk, and high-risk probiotic [7]. The 15 strains that were identified as low-risk probiotics can be prioritized for potential further studies for clinical application. These results provide understanding of F. prausnitzii as a future probiotic, especially for CD patients.
Probiotic Treatment: F. prausnitzii Case Study
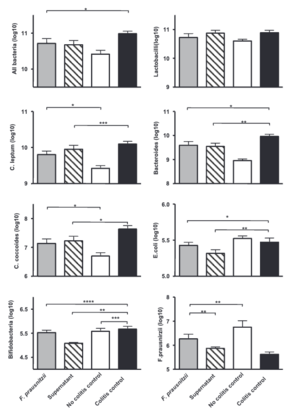
Many different studies point to using F. prausnitzii as a potential probiotic treatment for CD and Ulcerative Colitis (UC). One study investigated the effects of live F. prausnitzii and its supernatant, demonstrating a significant reduction in colitis severity, a condition related to Crohn’s that affects the intestines [14]. There was a significant improvement in severity scores as well as decreased weight loss and restoration of colon lengths. Moreover, F. prausnitzii and its supernatant positively influenced the balance of gut bacteria [14]. Treatment with live F. prausnitzii or its supernatant improved the gut microbiota balance, nearly normalizing levels of healthy gut bacteria such as C. leptum, C. Coccoides, and Bacteroides that are all disrupted by the disease, but then restored when treated with F. prausnitzii [14].
Notably, not only did the severity of the disease decrease, F. prausnitzii acted as a probiotic in the mice by restoring the healthy gut bacteria as well as increasing the abundance of F. prausnitzii. Low amounts of F. prausnitzii were found to be associated with endoscopic recurrence at 6 months with anti-inflammatory effects suggested in vitro as well as in vivo. These effects were confirmed in vivo on TNBS-induced colitis, with mice being treated with either F. prausnitzii or the supernatant [14]. TNBS otherwise known as trinitrobenzene sulfuric acid is an experimental compound that induces inflammation in the gut in order to resemble Crohn’s disease in studies [14]. Using this method helps to study the pathophysiology of the diseases and look to potential therapeutic treatments.
The mice that were treated with F. prausnitzii had a protective effect for the mice. These mice had a reduced IL-12 and a lower IL-10 colonic amount compared to the colitis control [14]. IL-12 is responsible for regulating the immune response promoting Th1 cell differentiation and activation. Elevated levels are induced by prolonged inflammation. Furthermore, Il-10 is an anti-inflammatory cytokine that suppresses the immune response [14]. In this study there is shown to be lower levels of IL-10 pointing to the fact that F. prausnitzii helps to lower the anti-inflammatory response, making the need for IL-10 cytokines to be fewer than that of the disease control [14]. This imbalance was due to the counterbalances of F. prausnitzii acting to lower the inflammation [14].
F. prausnitzii as a probiotic could be further investigated as it was tested on a TNBS-induced colitis model and showed significantly positive results. However, butyrate presence was not taken into consideration. Addressing dysbiosis using F. prausnitzii as a promising probiotic, can be a really good strategy for the future in further CD treatments. Although this study points researchers in a very positive direction, this study poses some limitations. Clinical research is needed to establish diagnostic tools to determine what the ideal patient population is for this probiotic treatment (F. prausnitzii) and whether this is better in patients with low levels of Firmicutes. Each patient can have different severities and symptoms for CD, especially with certain genetic predispositions. Further studies are essential to determine the efficacy and suitability of this potential treatment in different patient cohorts.
Limitations and Future Research
There is no standardized process for diagnosing patients with Crohn’s disease. In future studies, researchers are looking to find specific markers to diagnose Crohn’s disease early on in order to fully understand and account for all of the factors that contribute to the disease. In order to help this, there needs to be more studies that focus on specific indicators in the body such as antibodies in the blood, along with genetic distinctions which could help indicate the severity of the disease. By doing this, it can help to further understand the specific dysbiotic patterns and their implication for treatment response and they are yet not fully understood. Future research can be done in order to figure out who is most susceptible to Crohn’s Disease in order to see if screening patients before they acquire symptoms is beneficial. As Crohn’s is a chronic disease that is interconnected and multi-faced with many different factors, finding the right group of patients to screen would be difficult however crucial in understanding the other potential factors of the disease. However, there is not enough evidence to screen patients only with a few risk factors. In summary, as there is growing evidence on the implications of F. prausnitzii in the pathogenesis of CD patients and a therapeutic treatment, there are more studies that can be conducted in order to find the maximum clinical effect the bacteria can have on improving patients with CD.
Conclusion
Research proves the connection between the human gut microbial community and Crohn’s Disease. There is less literature on the link between, exact F. prausnitzii strains, but enough is present to continue to research the correlation between the two developing further research on the topic. Crohn’s Disease is a non-treatable illness that causes significant interference in the lives of those inflicted with it and by moving forward with trying to work to create a treatment, the factors contributing to the disease need to be understood. The effect of F. prausnitzii, on the gut microbiota initially demonstrates the formation of this relationship with the overlap in F. prausnitzii levels, affecting CD patients. In short, the dysregulation of gut microbiota affects humans well being and Crohn’s disease is one that it causes to come to fruition. With all of this information, we can look into more possible clinical trials using F. prausnitzii as a potential probiotic treatment. Further research is needed to reach these steps, but as more is learned and developed about the connection between the gut-microbiota and bacteria F. prausnitzii, and Crohn’s disease, many scientific discoveries can be established.
References
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Crohn’s Disease - Harvard Health.” Harvard Health Publishing, 15 Nov. 2018. Retrieved April 9, 2024, from https://www.health.harvard.edu/a_to_z/crohns-disease-a-to-z
- ↑ 2.0 2.1 2.2 2.3 Mayo Clinic (2022). Crohn’s Disease - Symptoms and Causes. [online] Mayo Clinic. Available at: https://www.mayoclinic.org/diseases-conditions/crohns-disease/symptoms-causes/syc-20353304
- ↑ Andrew, M. and Karpatkin, M. (1982). A simple screening test for evaluating prolonged partial thromboplastin times in newborn infants The Journal of Pediatrics, 101(4), pp.610–612. doi:https://doi.org/10.1016/s0022-3476(82)80721-x
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Slivinski, N. (2022). Gut Bacteria and Crohn’s Disease. [online] WebMD. Available at: https://www.webmd.com/ibd-crohns-disease/crohns-disease/crohns-disease-gut-bacteria
- ↑ Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K.S., Manichanh, C., Nielsen, T., Pons, N., Levenez, F., Yamada, T., Mende, D.R., Li, J., Xu, J., Li, S., Li, D., Cao, J., Wang, B., Liang, H., Zheng, H. and Xie, Y. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature, 464(7285), pp.59–65. doi:https://doi.org/10.1038/nature08821
- ↑ 6.0 6.1 6.2 6.3 6.4 Moore, W.E. and Moore, L.H. (1995). Intestinal floras of populations that have a high risk of colon cancer. Applied and Environmental Microbiology, 61(9), pp.3202–3207. doi:https://doi.org/10.1128/aem.61.9.3202-3207.1995
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 Gupta, D., Shah, H.P., Malu, K., Berliner, N. and Gaines, P. (2014). Differentiation and Characterization of Myeloid Cells. Current Protocols in Immunology, [online] pp.22F.5.1–22F.5.28. doi:https://doi.org/10.1002/0471142735.im22f05s104
- ↑ 8.0 8.1 8.2 8.3 8.4 Martín, R., Rios-Covian, D., Eugénie Huillet, Auger, S., Sajad Khazal, Luis Bermudez Humaran, Sokol, H., Chatel, J.-M. and Langella, P. (2023). Faecalibacterium: a bacterial genus with promising human health applications. Fems Microbiology Reviews, 47(4). doi:https://doi.org/10.1093/femsre/fuad039
- ↑ 9.0 9.1 9.2 9.3 9.4 Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermudez-Humaran, L.G., Gratadoux, J.-J. ., Blugeon, S., Bridonneau, C., Furet, J.-P. ., Corthier, G., Grangette, C., Vasquez, N., Pochart, P., Trugnan, G., Thomas, G., Blottiere, H.M., Dore, J., Marteau, P., Seksik, P. and Langella, P. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proceedings of the National Academy of Sciences, 105(43), pp.16731–16736. doi:https://doi.org/10.1073/pnas.0804812105
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 Zhou, L., Zhang, M., Wang, Y., Dorfman, R.G., Liu, H., Yu, T., Chen, X., Tang, D., Xu, L., Yin, Y., Pan, Y., Zhou, Q., Zhou, Y. and Yu, C. (2018). Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflammatory Bowel Diseases, [online] 24(9), pp.1926–1940. doi:https://doi.org/10.1093/ibd/izy182
- ↑ Rashid, T., Ebringer, A. and Wilson, C. (2013). The Role ofKlebsiellain Crohn’s Disease with a Potential for the Use of Antimicrobial Measures. International Journal of Rheumatology, [online] 2013, pp.1–8. doi:https://doi.org/10.1155/2013/610393
- ↑ 12.0 12.1 Dempsey, E. and Corr, S.C. (2022). Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Frontiers in Immunology, [online] 13, p.840245. doi:https://doi.org/10.3389/fimmu.2022.840245
- ↑ National Center for Complementary and Integrative Health (2019). Probiotics: What You Need To Know. [online] NCCIH. Available at: https://www.nccih.nih.gov/health/probiotics-what-you-need-to-know
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 14.8 Maioli, T.U., Borras-Nogues, E., Torres, L., Barbosa, S.C., Martins, V.D., Langella, P., Azevedo, V.A. and Chatel, J.-M. (2021b). Possible Benefits of Faecalibacterium prausnitzii for Obesity-Associated Gut Disorders. Frontiers in Pharmacology, 12. doi:https://doi.org/10.3389/fphar.2021.740636
