Cyanobacteria and Cyanotoxins
Introduction
By Mark Boniface
Cyanobacteria are a group of bacteria that harness energy through the process of photosynthesis, absorbing the sun’s energy and converting it into usable energy. These bacteria originally got their name from their bluish-green color. Cyanobacteria are important in many ecosystems, as they produce gaseous oxygen as a product of their photosynthetic process. These bacteria and their descendants are credited with the production of all the oxygen gas on Earth. Since their origin, cyanobacteria have initiated a massive blooming of biodiversity of aerobic organisms, and caused the near-extinction of oxygen-intolerant organisms.
Along with the production of essential and highly demanded oxygen gas and the fixing of carbon dioxide and nitrogen, cyanobacteria also produce a variety of toxins. These harmful agents are primarily neurotoxins, cytotoxins, endotoxins, or hepatotoxins. Cyanobacteria produce these toxins to kill off other local species, giving themselves more room for growth. These toxins arise when environmental conditions are optimal for cyanobacteria to reproduce explosively and exponentially, resulting in algal blooms. These blooms are not only harmful for many aquatic species, but also for humans who ingest contaminated fish and shellfish or swim in or are exposed to contaminated water. It is thought that cyanobacteria are an environmental cause of degenerative neurological diseases such as Amyotrophic Lateral Sclerosis (ALS), Parkinson’s disease, and Alzheimer’s disease[1].
Cyanobacteria have also been identified as a potential source of renewable energy due to their ability to produce sugars and ethanol as well as agents with potentially antibiotic properties.
Cell Structure
Cyanobacteria have a typical cell structure for gram-positive cells, featuring a thick peptidoglycan layer that lies between the cell membrane and outer membrane. This layer is what allows many microbes to be stained purple by dyes that are used to make bacteria visible. Just inside the cell wall, cyanobacteria have layers of thylakoids, which are membrane-bound structures where the “light” photosynthetic reactions occur. Each thylakoid is covered with phycobilisomes which act like antennae that harvest light. Thylakoids are often present as stacks of disc-like structures, called grana, which allow maximum capturing of light energy. The grana are interconnected by smaller thylakoids which allow each stack of discs to function as a single unit. Buried within the layers of thylakoids are structures called cyanophycin, which possibly function as nitrogen and carbon storage. The thylakoids are located near the wall of the spherical cells; further toward the interior of cyanobacteria, thylakoids are replaced by ribosomes. Finally, at the center of the cell lies the DNA of the cell as well as the cyanobacteria’s carboxysomes. These are bacterial microcompartments that hold the RuBisCO enzymes, which are responsible for fixing carbon dioxide. RuBisCO is tethered to the inside of these carboxysomes. This arrangement is beneficial to the cell since the enzyme carbonic anhydrase is also held inside these compartments, and these two enzymes together use substrate channeling, the passing of a product of one enzyme directly to the next enzyme. This arrangement increases RuBisCO’s carbon fixation efficiency[2].
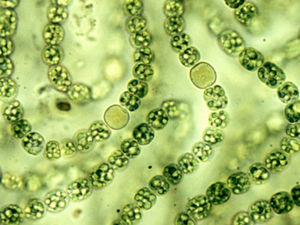
Cyanobacteria lack flagella, making it difficult for them to move under their own power. However, some species can move by gliding along surfaces. This movement requires contact with a solid surface such as a rock face, and occurs in the direction that is parallel to the longest axis of the cell or filament. Special small protein fibers and other organelle-like structures allow this gliding movement to occur. This process is thought to be due to the secretion of a mucilage during movement, but the exact mechanism of gliding is still unknown[3]. Some species of cyanobacteria such as Oscillatoria form multicellular films that are capable of movement through use of oscillating motions. Other species move through flotation by forming gas vesicles bound by protein sheaths or by forming long filamentous chains with other cyanobacteria as seen in figure 1. This figure shows the cyanobacterial species Nostoc formed into a long chain with heterocysts, specialized nitrogen fixing cells.
Photosynthesis
Traditionally, cyanobacteria have been connected to the plant kingdom as the predecessor to the chloroplast, but recently they have been separated due to several unique features. First, they are capable of fixing carbon. Cyanobacteria use the energy of captured sunlight to drive photosynthesis, the process of splitting water molecules into oxygen, protons, and electrons. Since cyanobacteria are typically found in all marine environments, they tend to employ “carbon concentrating mechanisms” to help acquire carbon in the form of carbon dioxide or bicarbonate. To accomplish this, cyanobacteria use carboxysomes that were previously mentioned. These structures hold the carbon fixing enzyme RuBisCO. The cyanobacteria use metabolic channeling to increase local carbon concentrations and make RuBisCO more efficient. Unlike eukaryotic cells, cyanobacteria lack compartmentalization of the thylakoid membrane which runs parallel to the plasma membrane. This prevents the sharing of energy carrier pools such as cytochrome c and ferredoxins and inhibits interactions between photosynthetic and respiratory metabolism. There is a great amount of diversity in cyanobacterial species’ respiratory systems; this diversity is called the “branched electron transport chain.”
Cyanobacteria do not have any internal membranes or nuclei, but do have many folds on their external membrane that function similarly to the way an internal membrane would in the mechanism of photosynthesis. The bluish-green chlorophyll accessory pigment phycocyanin functions to capture light energy from the sun, and then uses the surrounding water as an electron donor to produce oxygen gas as a byproduct. The captured carbon dioxide or bicarbonate is reduced by taking electrons from the water and donating it to the carbon. This forms carbohydrates through the Calvin cycle, the same cycle used by eukaryotic cells.
Cyanotoxins

Cyanobacteria are one phylum of microbes that have the ability to explosively reproduce given the proper environmental conditions. The end results of these periods of high growth are algal blooms. These blooms can potentially cover hundreds of square miles and are often seen by satellite imagery, like in Figure 2, and while the individual cyanobacteria live only a few days, the blooms tend to last a few weeks. These large fields of organisms look like a blanket of foam or scum, often bright green in color due to the chlorophyll of the bacteria. They can also appear blue or even a brownish-red depending on the species present. For both saltwater and freshwater systems, algal blooms are most often an ecosystem’s response to a large addition of natural or artificial substances such as phosphates and nitrogen in the form of detergents, fertilizers, or even sewage. This process is called eutrophication. While the majority of eutrophication events are artificially driven, they can happen naturally but over a much longer time period and to a much less extreme extent. The presence of high levels of phosphate and nitrogen allow rapid plant growth and decay, mainly of simple algae which can quickly take up the available resources and prevent more complex plant life take root in the new phosphate rich environment. However, the period of high growth must eventually end, so as the phosphates are consumed and growth slows, most, if not all, of the cyanobacteria die and begin to decay. This process consumes oxygen, which in turn makes the localized area a hypoxic dead zone, killing any marine life present. Despite this, the majority of these blooms are harmless; however, this is not always so. Sometimes harmful algal blooms arise, often called “red tides” or harmful algal blooms, called HABs for short. These tides contain toxins and pathogens which kill marine life and are potentially fatal for humans[4]. Altogether, over 84 cyanotoxins are known to exist, but only a very small number have been studied[5]. The most common forms that these toxins take are as alkaloids, amino acids, cyclic peptides, and polyketides.
Alkaloid Cyanotoxins
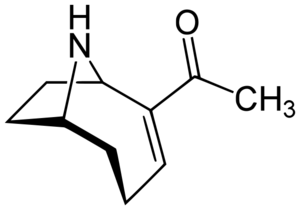
Alkaloids are naturally occurring compounds which contain nitrogen atoms and are produced by many organisms, including cyanobacteria. When animals are exposed to alkaloids, there are often psychotropic and toxic effects. The most famous among the alkaloid cyanotoxins is anatoxin-a. Anatoxin-a was first discovered in Saskatchewan, Canada in 1961 when a herd of cows suddenly died after drinking water from a lake in which there was an active algal bloom[6]. This toxin was first termed as the “Very Fast Death Factor” and is produced by at least four species of cyanobacteria and can be found across North America, Europe, Africa, Asia, and New Zealand.
After an organism is exposed to anatoxin-a, the effects progress very quickly. Nerve cells are affected initially, causing a rapid loss of coordination and muscle spasms and twitching, followed by convulsions and seizures and then ending in death due to paralysis of the respiratory system. This process is termed “flaccid paralysis” due to the lack of trauma or other cause of paralysis, as well as because of the characteristic calmness of patients, who are generally conscious throughout the paralytic process. Anatoxin-a, whose chemical structure is shown in Figure 3, works by fitting inside the nerve receptors of muscle cells that are usually filled by a neurotransmitters, causing the muscles to contract. However, once anatoxin-a binds to this receptor, it does not unbind, preventing muscles from relaxing and returning to the resting state, inducing gradual paralysis. Anatoxin-a is the most widely researched cyanotoxin due to its interactions with the nervous system and its deadliness as a neurotoxin, however, the lethal dose of anatoxin-a in humans is unknown. An experiment performed by Araoz et al. showed that in mice, the lethal dose of exposure of anatoxin-a was around 25 mg per kilogram body weight, and the toxin’s effects were seen between two and five minutes after exposure. It has been theorized that these times could be similar in humans[7]. Anatoxin-a is produced by several genera of cyanobacteria including Anabaena, Aphanizomenon, and Microcystis.
There are several other known alkaloid cyantoxins produced by cyanobacteria that have been identified and researched. Cylindrospermopsin (commonly abbreviated to CYN or CYL) was first discovered in Australia on Palm Island at the end of a disease outbreak. The disease was traced back to an algal bloom of the cyanobacteria Cylindrospermopsis raciborskii in the local supply of drinking water where a toxin was found and later identified as cylindrospermopsin. This was one of widespread discoveries of cyanotoxins. CYN and the cyanobacteria that produce it were later found in tropical and subtropical water bodies in Australia, Europe, Israel, Japan, and the United States. This toxin is categorized as a hepatotoxin and targets the liver and kidney tissues. CYN is suspected to work by inhibiting the synthesis of proteins, but it is unclear how exactly the mechanism interacts with the cells of the liver. The contamination of drinking water supplies is not the only concern raised by CYN. Cylindrospermopsin is a concern due to the way CYN accumulates in freshwater organisms who feed off of the cyanobacteria, building up large enough amounts of CYN in animal bodies that eventually make their way to humans who consume these organisms and could become poisoned[8]. Another alkaloid cyanotoxin of note is one of the most potent natural toxins known, saxitoxin (STX). STX is produced by several species of cyanobacteria: Anabaena sp., Aphanizomenon sp., Cylindrospermopsis sp., Lyngbya sp., and Planktothrix sp[9]. This is the same neurotoxin produced by some species of puffer fish and clams. STX accumulates in shellfish and certain types of fish, and upon consumption by humans, can cause paralytic shellfish poisonings. STX induces paralysis by inhibiting sodium channel activity in a similar manner to anatoxin-a, causing muscles to become active and never rest, causing respiratory paralysis.
BMAA
BMAA (beta-methylamino-L-alanine) is a non-proteinogenic amino acid that is produced by cyanobacteria in both soil and marine environments, freshwater and saltwater. The genera that produce the BMAA toxins are Anabaena, Planktothrix, and Nostoc, among others. Terrestrially, BMAA has been found in plants exhibiting symbiotic characteristics such as lichens and some species of ferns. It is thought that BMAA is a potential environmental risk for many degenerative neurological diseases such as ALS, Parkinson’s disease, and Alzheimer’s disease, but the exact mechanisms of interaction is unknown.
Cyclic Peptides
Cyanobacteria also produce several toxins that take the form of peptides, short chains of amino acids linked by peptide bonds similar in structure to that of proteins. The overall structure of each of the cyanobacteria produced peptides are cyclic in shape. This property of the peptides makes them difficult to break down and metabolize by the body when ingested, causing them to accumulate in the liver of animals and humans. This is where the toxins take effect, damaging liver tissues and promoting tumor growth in the liver. The two most known cyclic peptide hepatotoxins are classified as microcystins and nodularins. Microcystins are produced by many cyanobacterial species and there are thought to be around fifty to sixty variants of microcystins. While not seeming as dangerous as neurotoxins like anatoxin-a, the hepatotoxins are of the most concern to humans since they can accumulate in fish and shellfish in areas affected by algal blooms and are a common cause of hepatotoxic shellfish poisoning. The chief microcystin causing the poisonings is microcystin-LR.
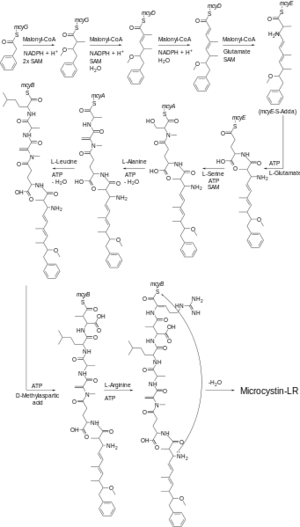
Microcystin-LR is synthesized by Microcystis aeruginosa, a species of freshwater cyanobacteria. The synthesis of microcystin-LR occurs in a complicated biosynthetic pathway depicted shown in Figure 4. Microcystin-LR works as a toxin by inhibiting protein phosphatase. Particularly types 1 and 2A protein phosphatase in the cytoplasm of liver cells. This inhibition causes an increase to the amount of phosphorylation of proteins occuring in the liver cells. Microcystin-LR accomplishes this by binding directly to the main catalysis site in the phosphoprotein phosphatase enzymes, blocking access of substrates to the active site and fully inhibiting the enzyme. This results in the buildup of phosphorylated proteins in the liver and ends in hepatotoxy[10]. Microcystin-LR is toxic to both humans and animals although there no confirmed reports of human death due to poisoning by microcystin-LR, however, there are reports of sickness and injury caused by it. But there are confirmed cases of deaths due to exposure to other, less prominent, microcystins. The most notable case of microcystin poisoning was in Brazil in 1996 where 116 patients reported experiencing multiple symptoms such as visual disturbances, nausea, vomiting, and general weakness. One hundred of these patients also experienced acute liver failure[11]. The other main type of cyclic peptide cyanotoxins are called nodularins. These toxins are found in algal blooms in salt waters throughout the world. The primary and most common of these nodularins is nodulin-R, another cyclic nonribosomal peptide[12]. These blooms most often occur in the Baltic Sea in the late summer and have similar hepatotoxic properties to that of the microcystins.
Control of Algal Blooms
Since the recognition of algal blooms as environmental hazards to marine life and potentially humans, several chemical controls have been discovered and developed to be deployed into the algal blooms if the need arises. The chemical compounds are calcium hypochlorite, copper sulfate, cupricide, and simazine. These chemicals are required in large amounts and need to be deployed several times to successfully halt the progress of an algal bloom. However these compounds present with their own risks, copper sulfate in particular has many toxic properties when marine and terrestrial life is exposed to it. Cupricide is also toxic, but to a lesser extent so it is often the choice for algal bloom control along with Simizane, an herbicide that will defeat the algal bloom and continue to kill off any growth in the area for several days past application.
Research and Biotechnology
Unicellular cyanobacteria are an important model organism for research for its effects on larger organisms’ nervous systems as well as a pathway to renewable energy through its means of converting sunlight into usable energy. Research is underway using cyanobacteria to produce combustible fuels. A company called Algenol have cultured a genetically modified cyanobacteria which they keep in seawater inside a clear plastic enclosure. These cyanobacteria then use photosynthesis to produce sugars and pyruvate from the available carbon dioxide and water. Soon the cells in the enclosure begin to excrete ethanol into the sea water. The ethanol then evaporates out of the water due to the solar energy and builds up on the ceiling of the enclosure. As the vapors form droplets, they travel down the enclosure’s walls into ethanol collectors and is transported to a facility which further purifies it into usable fuel. In March 2013, Algenol claimed that its facility in Florida was able to achieve yields of around 9000 gallons per acre per year of ethanol[13]. Algenol plans to expand and is planning to meet the United States' demand for ethanol in gasoline by the year 2025, assuming the amount of ethanol used in gasoline stays the same. Using cyanobacteria to produce ethanol is beneficial since it requires one tenth the area when compared to biomass sources such as corn, and only uses some fresh water.
Other areas of research have identified that some seaweeds and cyanobacteria to possess anti-inflammatory compounds and antibacterial agents. Off of the Kona Coast in Hawaii, an algal bloom left the local reef partially bleached of color and there was a large increase in the amount of seaweed growing in the area. Samples were taken by divers and after preliminary tests were run on the seaweeds, it was found that seaweeds (the cyanobacteria Leptolyngbya crossbyana) were producing natural products called honaucins which possessed potent anti-inflammatory and bacteria-controlling properties. These substances prevented bacteria from “swarming” on surfaces like the seaweeds themselves, limited areas in which bacteria could grow and halting the quorum sensing factor of bacteria[14]. This is significant because this ability to stop quorum sensing could translate into a potential cure and prevention for bacterial infections.
Conclusion
Cyanobacteria are gram-positive bacteria that are universally present in many ecosystems. These small cells are credited with producing the vast majority of the breathable oxygen on the planet and gave rise to the era of organisms who rely on aerobic respiration. Cyanobacteria are most commonly found in both freshwater and saltwater where they can form large algal blooms covering hundreds of square miles in response to exposure to phosphates and nitrogen. These algal blooms eventually decay, leaving behind a severely hypoxic environment which can in itself kill marine life. Sometimes these algal blooms are not harmless and become homes to many pathogens and toxins. These cyanobacteria produce hepatotoxins and neurotoxins which can kill marine life, animals, and even humans. These toxins can injure swimmers in toxic waters or can build up in fish and shellfish which are then consumed by humans. These patients then can develop acute liver failure or expire from respiratory paralysis. While not always harmful, algal blooms are concern worthy and steps to control their development are in place. Chemical agents that can be dispersed onto growing algal blooms can halt their growth and limit the damage the bloom could cause. Cyanotoxins and their effects are not fully understood, and neither are the organisms that create them. Recently studies of cyanobacteria have identified them as a possible source of renewable energy in the form of ethanol production as well. Medically they are a pathway to learning more about interactions of neurons and muscles as well as a potential source for new antibiotic agents to help cure many infections which are resistant to the available means of treatment.
References
[13] "Algenol Biofuels exceeds 9,000 gallons of ethanol per year per". Algenol Biofuels. 6 March 2013.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2015, Kenyon College.
