Desulfobulbus propionicus
Classification
Domain Bacteria; Phylum Proteobacteria; Class Deltaproteobacteria; Order Desulfobacterales; Family Desulfobulbaceae
Species
Desulfobulbus propionicus
Description and Significance
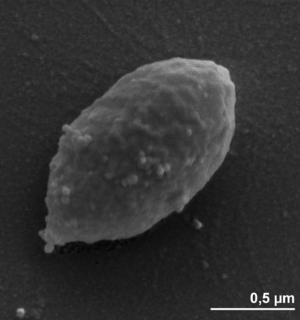
Desulfobulbus propionicus is a sulfate-reducing, Gram-negative bacteria that plays a key role in the sulfur cycle [4]. Currently, there are three described strains: 1 rp 3 (designated as the type strain), 2 pr 4, and 3 pr 10 [1]. Its appearance is ellipsoidal with pointed ends, and cells are roughly 1-1.3 by 1.8-2 micrometers in size [4]. D. propionicus is described as non-motile, surrounded by pili, and lacking a flagellum [1]. The two other closely related strains, 2 pr 4 and 3 pr 10, are motile by a single polar flagellum [1]. The type strain cannot actively propel itself, it must rely on gliding motility via solid surfaces [1]. One of the important characteristics of D. propionicus is that it can both create and degrade propionate [2].
It can typically be found in anaerobic freshwater mud, brackish or marine habitats, and manure deposits [3]. They also live in the intestines of animals like cows. Most members of the family Desulfobulbaceae are mesophilic, some are psychrophilic [3].
Genome Structure
D. propionicus has a single circular chromosome that is 3,851,869 base pairs long. A majority of the genome is composed of guanine + cytosine (58.9%). It contains a total of 3,408 genes, 3,351 of which are protein-coding genes, and 57 of which are RNA genes. Roughly 2,402 of these genes are predicted to have a function [4].
Cell Structure, Metabolism and Life Cycle
Characteristic of Gram-negative bacteria, the cell wall of D. propionicus contains a thin peptidoglycan layer. Within its cytoplasm are enzymes and proteins essential for anaerobic metabolism [4]. The cell membrane and cytoplasm contain b- and c- type cytochromes, a distinguishing feature of the type strain from its closely related strains [4].
As mentioned earlier, D. propionicus is a sulfate-reducing bacteria, described as a strict anaerobic chemoorganotroph under typical conditions [3]. However, it has more recently been found to grow in aerobic environments while maintaining its ability to oxidize sulfide, elemental sulfur, sulfite, and polysulfide to sulfate, but this is not a widely recognized characteristic [4]. Thiosulfate is also formed from elemental sulfur [4]. Sulfate, sulfite, and thiosulfate all serve as electron acceptors, reducing to hydrogen sulfide. Propionate, lactate, pyruvate, ethanol, and propanol are all used as electron donors and carbon sources, ultimately being oxidized to acetate as the end-product of incomplete oxidation [4]. In the absence of sulfate, D. propionicus can perform fermentation, producing organic acids like acetate and propionate in a 2:1 ratio as byproducts [4].
Via the methylmalonyl-CoA pathway, D. propionicus produces two acetates and one propionate from three pyruvates [4]. It has also been found to reduce iron, Fe(III), to sustain growth. Iron acts as a catalyst to increase the efficiency with which D. propionicus produces sulfate [4].
D. propionicus and other members of Desulfobulbaceae do not form spores [4]. Members of this family reproduce asexually via binary fission, resulting in two genetically identical daughter cells [4].
Ecology and Pathogenesis
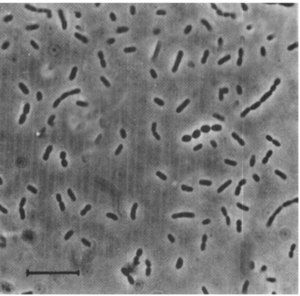
D. propionicus is free-living and can be found single, in pairs, or in short chains [1]. The optimal pH range for D. propionicus is 7.1-7.5, but it can survive and grow between 6.0-8.6 [1]. As for temperature, it can survive between 10-43 degrees Celsius while the optimum temperature for growth is 39 degrees Celsius [1]. These optimum conditions may vary among the other strains [1]. As mentioned above, it can typically be found in anaerobic freshwater mud, brackish or marine habitats, and manure deposits [3].
Being sulfate reducers, D. propionicus and other members of Desulfobulbaceae play key roles in the sulfur cycle. Their population growth influences sulfate reduction because their reproduction is linked to their metabolic activities [3].
D. propionicus is not typically associated with human diseases [1]. It is primarily an environmental bacteria that’s involved in biogeochemical processes, namely sulfate reduction, and is not considered pathogenic.
References
[3]Kuever, J. ““The Family Desulfobulbaceae.” “The Prokaryotes”. 2014. P. 75-86.
Author
Page authored by Samantha Zullo, student of Prof. Bradley Tolar at UNC Wilmington.