Ectomycorrhizal symbiosis
Introduction
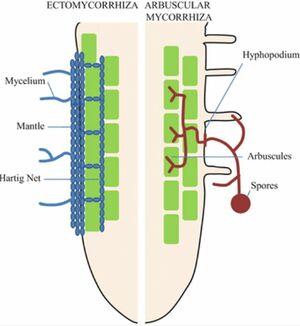
An ectomycorrhiza is a mutualistic relationship occurring between fungi and the root systems of certain host plants. Unlike other mycorrhizae, ectomycorrhizae aid their hosts by creating networks of interlacing hyphae that surround instead of penetrate the epidermal and cortical cells of host roots. [1][2] These networks, known as Hartig nets, mediate the exchange of growth-limiting nutrients and photosynthate between fungi and plants. This mutualistic exchange is essential to the function of 80-90% of all temperate and boreal forest trees, influencing soil processes, nutrient cycling, plant health, matter decomposition, and carbon sequestration through the repression of soil respiration. [3][4] In nutrient-limited environments, ECM fungi provide a range of benefits for their hosts, such as drought and salinity tolerance[5], and nutrient acquisition by accessing nitrogen beyond the nutrient depletion zone of the host. ECM fungi can be found throughout the world in several climate zones in association with the roots of the families birch, willow, beech, pine, as well as dipterocarp, rose, and myrtle.[1]
Nutritional Exchange
Uptake of Nitrogen
Ectomycorrhizal (ECM) fungi can uptake and provide a host with a wide range of macronutrients such as potassium, phosphorus, sulfur, and micronutrients such as iron, zinc, and copper. However, they are most recognized for the transport of nitrogen (N) as it is the main growth-limiting factor in many forest ecosystems. [6] The first step of nutritional exchange between ECM fungi and a plant host is for encoded N transporters to uptake and utilize nitrate and ammonium from the soil. The utilized N can be acquired from either inorganic or organic N sources. Ammonium can be taken up from inorganic sources and is the most preferred source by ECM fungi as it does not require energy to be used on chemical reduction. [1]
Three main ammonium transporters have been identified in several fungal ECM species: AMT1, AMT2, and AMT3. The former two are high-affinity transporters, meaning their expression is upregulated most in conditions of low ammonium. [7] Nitrate can also be uptaken by ECM fungi and requires the regulation of nitrate transporters, such as LbNRT2, and nitrate reductase enzymes. [8] In the presence of ammonium, the nitrate uptake pathway is downregulated due to the fungal preference for ammonium. The oxidative decomposition mechanisms inherited by ECM fungi from their saprotrophic ancestors allow for organic N sources to be used as well. Peptidase secretion is then used to reduce proteins from the soil into smaller peptide products. [1]

Nitrogen and Carbon Transfer
After uptake to the fungal mycelium, nitrogen can be used in various ways by fungi. Some of the nitrogen is metabolized by the ECM fungi itself, while most is stored in cells or sent to fungal root tips to be transferred to a host plant. [9] Once in the root tips, N has to cross two membranes to be imported to host cells. The process of nitrogen transfer across the plant-fungal interface requires a system of coordination in the expression of fungal and host N transporters, as well as aquaporins[10] and voltage-dependent cation channels.[11]
Separately, hosts transport a third or more of their photosynthate to ECM fungi. [12] This carbon is transferred in the form of sugars into the apoplastic space between plant and fungal cells.[13] In order to execute this, plants encode sucrose transporters for long-distance transport within their own cells [14] and transfer sucrose cleavage products using monosaccharide transporters (SWEET transporters). [15] While ECM fungi do express monosaccharide transporters, sucrose has been found to be an invalid nutrient as they lack expression of sucrose transporters and invertases. This means ECM fungi rely on their hosts to cleave photosynthate into usable monosaccharides. [16]
Nutrient Quantity Regulation
A consensus on what moderates the carbon-nitrogen flux in ectomycorrhizae is not yet accepted, unlike carbon-phosphorus flux in arbuscular mycorrhizae.[17] One theory explaining flux regulation in ECM is the concept of “reciprocal rewards.” This proposes carbon and nitrogen transfer are one bidirectional system and that plants preferentially transfer more photosynthate to fungi that have provided the largest quantity of nutrients. Reciprocal rewards would allow for a more stable system of mutualism with selection for the fungi most efficient in nitrogen transfer.[18] Studies observing Pinus muriata-Suillus brevipes[19] and Fagus sylvatica associated fungi support this theory, demonstrating that nitrogen-enriched soil “hotspots” of ECM fungal mycelium are enriched with more carbon.[20]
However, other studies have results suggesting that N/C flux is neither reciprocal nor related. Pinus pinaster trees have been found to transfer C to their symbionts when it is excessively produced and continue that transfer even when the quantity of N received is lowered.[21] Also, when trees in N-limited boreal forests increase C allocation to fungi, they are not met with an increase in received N.[22] The mechanisms moderating C/N flux are still in dispute, but we know that under certain conditions C allocation may be decided by the N quantity transferred from ECM fungi.
Nitrogen Availability in Soil
Nitrogen depletion is known to be a main cause of growth limitation in many forest ecosystems. Some studies have shown that the addition of ECM fungi to forests can overcome nitrogen-driven growth limitations.[24] However, other evidence suggests that these fungi may enhance N limitation as they themselves utilize N in growth, and C allocation only heightens usage. This may promote ECM fungi to “hoard” N in forest ecosystems and further soil N limitation. [25] The persistence of ECM fungi may be partly explained by this limitation as an increased N concentration in soil leads to decreased species richness, plant colonization levels, mycelial growth, and sporocarp production. [26]
Genetics
Ectomycorrhizal fungi can be categorized as a diverse group of Basidiomycetes and include species that evolved independently from 60-80 saprotrophic ancestors. This diversity of genetics is shown in the variety of climates, hyphal growth, and nutrient storage between ECM fungi. [1] Due to their symbiotic nature, significant proportions of genes in ECM fungi are responsible for primary and secondary metabolism, as well as cell signaling.[27]
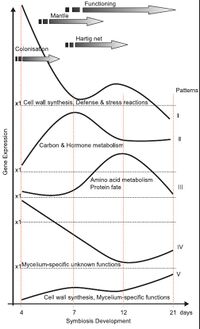
As ectomycorrhizal relationships are formed, gene expression is changed greatly in both fungi and host plants.[23] Studies monitoring gene expression through the developmental stages of Eucalyptus globulus-Pisolithus tinctorius ectomycorrhiza suggest that the genes upregulated most dramatically during symbiosis code for translation initiation factors, translation elongation factors, hydrophobins, components of the ubiquitin-proteasome pathway, and stress proteins.[27] Nitrogen and carbon metabolism is also upregulated during mycorrhiza formation after 12 days of contact. In the later stages of development, many upregulated genes code for enzymes involved in glycolysis, the tricarboxylic acid cycle, amino acid and protein synthesis, hormone metabolism, and signal transduction.[23]
Conclusion
Species of ectomycorrhizal fungi have proven to play a crucial role in many forest ecosystems throughout the world because of the many benefits they provide for host plants and their role in global carbon storage.[3] The exchange of nitrogen and carbon between symbionts takes place through the use of compound-specific transporters that cross the plant-fungal cell interface. The upregulation of gene expression for metabolic processes, translation factors, and signal transduction is necessary for nutritional symbiosis to take place in the mycelial Hartig net. Many factors of ECM relationships are not yet certain and we do not currently know whether the flux of nutrients between fungi and their hosts is reciprocal or unrelated. Research of ECM fungi is of growing importance due to its significance in restoration, forestry, and agriculture.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Stuart, Emiko K., and Krista L. Plett. "Digging deeper: in search of the mechanisms of carbon and nitrogen exchange in ectomycorrhizal symbioses." Frontiers in plant science 10 (2020): 1658.
- ↑ Tedersoo, L., May, T and Smith, M. "Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages" 2009. Mycorrhiza 20:217–263.
- ↑ 3.0 3.1 Read, David J. "Mycorrhizas in ecosystems." Experientia 47, no. 4 (1991): 376-391.
- ↑ Brundrett, Mark. "Diversity and classification of mycorrhizal associations." Biological reviews 79, no. 3 (2004): 473-495.
- ↑ Hajiboland, Roghieh, Naser Aliasgharzadeh, Shirin Farsad Laiegh, and Charlotte Poschenrieder. "Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants." Plant and Soil 331, no. 1 (2010): 313-327.
- ↑ Read, David J., Jonathan R. Leake, and Jesus Perez-Moreno. "Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes." Canadian Journal of Botany 82, no. 8 (2004): 1243-1263.
- ↑ Javelle, Arnaud, Mélanie Morel, Blanca‐Rosa Rodríguez‐Pastrana, Bernard Botton, Bruno André, Anne‐Marie Marini, Annick Brun, and Michel Chalot. "Molecular characterization, function and regulation of ammonium transporters (Amt) and ammonium‐metabolizing enzymes (GS, NADP‐GDH) in the ectomycorrhizal fungus Hebeloma cylindrosporum." Molecular microbiology 47, no. 2 (2003): 411-430.
- ↑ Kemppainen, Minna J., Maria C. Alvarez Crespo, and Alejandro G. Pardo. "fHANT‐AC genes of the ectomycorrhizal fungus Laccaria bicolor are not repressed by l‐glutamine allowing simultaneous utilization of nitrate and organic nitrogen sources." Environmental microbiology reports 2, no. 4 (2010): 541-553.
- ↑ Nehls, Uwe, and Claude Plassard. "Nitrogen and phosphate metabolism in ectomycorrhizas." New Phytologist 220, no. 4 (2018): 1047-1058.
- ↑ Dietz, Sandra, Julia von Bülow, Eric Beitz, and Uwe Nehls. "The aquaporin gene family of the ectomycorrhizal fungus Laccaria bicolor: lessons for symbiotic functions." New Phytologist 190, no. 4 (2011): 927-940.
- ↑ Chalot, Michel, Damien Blaudez, and Annick Brun. "Ammonia: a candidate for nitrogen transfer at the mycorrhizal interface." Trends in plant science 11, no. 6 (2006): 263-266..
- ↑ Nehls, U., F. Göhringer, S. Wittulsky, and S. Dietz. "Fungal carbohydrate support in the ectomycorrhizal symbiosis: a review." Plant Biology 12, no. 2 (2010): 292-301.
- ↑ Hennion, Nils, Mickael Durand, Cécile Vriet, Joan Doidy, Laurence Maurousset, Rémi Lemoine, and Nathalie Pourtau. "Sugars en route to the roots. Transport, metabolism and storage within plant roots and towards microorganisms of the rhizosphere." Physiologia plantarum 165, no. 1 (2019): 44-57.
- ↑ Doidy, Joan, Emily Grace, Christina Kühn, Françoise Simon-Plas, Leonardo Casieri, and Daniel Wipf. "Sugar transporters in plants and in their interactions with fungi." Trends in plant science 17, no. 7 (2012): 413-422.
- ↑ Chen, Li-Qing, Bi-Huei Hou, Sylvie Lalonde, Hitomi Takanaga, Mara L. Hartung, Xiao-Qing Qu, Woei-Jiun Guo et al. "Sugar transporters for intercellular exchange and nutrition of pathogens." Nature 468, no. 7323 (2010): 527-532.
- ↑ Martin, Francis, Andrea Aerts, Dag Ahrén, Aurélie Brun, E. G. J. Danchin, F. Duchaussoy, Julien Gibon et al. "The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis." Nature 452, no. 7183 (2008): 88-92.
- ↑ Garcia, Kevin, Pierre‐Marc Delaux, Kevin R. Cope, and Jean‐Michel Ané. "Molecular signals required for the establishment and maintenance of ectomycorrhizal symbioses." New Phytologist 208, no. 1 (2015): 79-87.
- ↑ Casieri, Leonardo, Nassima Ait Lahmidi, Joan Doidy, Claire Veneault-Fourrey, Aude Migeon, Laurent Bonneau, Pierre-Emmanuel Courty et al. "Biotrophic transportome in mutualistic plant–fungal interactions." Mycorrhiza 23, no. 8 (2013): 597-625.
- ↑ Bogar, Laura, Kabir Peay, Ari Kornfeld, Julia Huggins, Sara Hortal, Ian Anderson, and Peter Kennedy. "Plant-mediated partner discrimination in ectomycorrhizal mutualisms." Mycorrhiza 29, no. 2 (2019): 97-111.
- ↑ Kaiser, Christina, Werner Mayerhofer, Marlies Dietrich, Stefan Gorka, Arno Schintlmeister, Siegfried Reipert, Peter Schweiger et al. "Reciprocal trade of Carbon and Nitrogen at the root-fungus interface in ectomycorrhizal beech plants." In EGU General Assembly Conference Abstracts, p. 15133. 2017.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
- ↑ Corrêa, A., R. J. Strasser, and M. A. Martins-Loução. "Response of plants to ectomycorrhizae in N-limited conditions: which factors determine its variation? Mycorrhiza18: 413–427." (2008).
- ↑ 23.0 23.1 23.2 Duplessis, Sébastien, Pierre‐Emmanuel Courty, Denis Tagu, and Francis Martin. "Transcript patterns associated with ectomycorrhiza development in Eucalyptus globulus and Pisolithus microcarpus." New Phytologist 165, no. 2 (2005): 599-611.
- ↑ Näsholm, Torgny, Peter Högberg, Oskar Franklin, Daniel Metcalfe, Sonja G. Keel, Catherine Campbell, Vaughan Hurry, Sune Linder, and Mona N. Högberg. "Are ectomycorrhizal fungi alleviating or aggravating nitrogen limitation of tree growth in boreal forests?." New Phytologist 198, no. 1 (2013): 214-221.
- ↑ Terrer, César, Sara Vicca, Bruce A. Hungate, Richard P. Phillips, and I. Colin Prentice. "Mycorrhizal association as a primary control of the CO2 fertilization effect." Science 353, no. 6294 (2016): 72-74.
- ↑ Lilleskov, Erik A., Timothy J. Fahey, Thomas R. Horton, and Gary M. Lovett. "Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska." Ecology 83, no. 1 (2002): 104-115.
- ↑ 27.0 27.1 Voiblet, Catherine, Sébastien Duplessis, Nathalie Encelot, and Francis Martin. "Identification of symbiosis‐regulated genes in Eucalyptus globulus–Pisolithus tinctorius ectomycorrhiza by differential hybridization of arrayed cDNAs." The Plant Journal 25, no. 2 (2001): 181-191.
Edited by Ronan Daly, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2021, Kenyon College.
