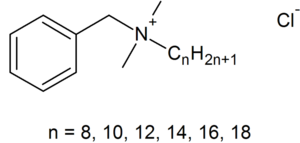Efficacy of antiseptic treatments
Introduction
Benzalkonium chloride (BAC) is a major non-alcohol-based active ingredient used for clinical, food line, and domestic household biocides [14,17]. A biocide is a general term for a chemical agent, that may be applied topically in/on living tissue (antiseptic) or on inanimate objects (disinfectant), in order to inhibit growth of (“-static”) or kill (“-cidal”) microorganisms [28].
Antiseptic hand sanitizers have been shown to subdue the prevalence of the common cold, acute respiratory syndromes, gastroenteritis, viral influenza, and more [1,13,15,20,21,22,24,25]. Similarly, they are important in limiting hospital-acquired (nosocomial) infections by Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSAs), and vancomycin-resistant Enterococcus (VREs) [4,8,12,14,16,23]. For such preventative measures, there are a variety of hand sanitizers available with alcohol-based and alcohol-free antiseptic agents including ethanol, triclosan and benzalkonium chloride [17,21]. Despite speculations of evolving resistance, BACs are extensively used biocides especially potent against enveloped microorganisms like Gram-positive bacteria and some viruses [14,17,18].
General Mode of Antiseptic Activity
Aside from the generality that antiseptics have less specific activities with multiple target sites compared to antibiotics, current studies on the mechanistic details of particular antiseptics remains largely inconclusive [16,18]. This broad level of activity is well summarized by the antiseptic first interacting with the cell surface, penetrating into the cell, then acting at intracellular target sites [18]. The details of each antiseptic depend on the biocide’s chemical nature, the pathogen, and the test conditions (eg. antiseptic concentration, pH, time of exposure, and temperature) [23]. Although antiseptic mechanisms lack mechanistic details, the general activity is still well agreed upon.
Quaternary Ammonium Compounds (QACs)
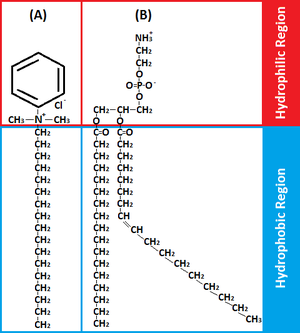
Benzalkonium chloride is classified as a Quaternary Ammonium Compound (QAC), NR4+ where R can be different carbon-hydrogen groups, and are a common antiseptic, because of theiramphiphilicity (hydrophobic and hydrophilic) [10,19]. BAC’s hydrophilic cationic region destabilizes the pathogen’s surface through electrostatic interactions with negatively charged components displacing surface-stabilizing cations [9,10,18]. Subsequently, BAC's hydrophobic region penetrates the hydrophobic bilayer to cause cell leakage [10,17,18]. The ultimate effect of BACs is to damage the pathogen’s membrane, and disrupt essential processes like ATP synthesis or solute uptake [18]. Therefore BAC’s amphiphilicity is critical in interacting with target membranes for efficacious antimicrobial action.
Susceptible Microorganisms
The key component of BAC’s antimicrobial activity is membrane destruction [10]. This is most effective against Gram-positive bacteria, some Gram-negative bacteria, some enveloped viruses, fungi, yeasts and protozoa due to varying surface structures [10].
Non-sporulating Gram-positive bacteria
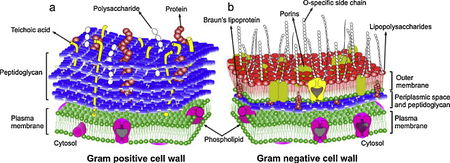
Staphylococcus aureus is a Gram-positive bacterium that can survive on antiseptic-free hands for at least 150 minutes, allowing it to spread and persist as a leading cause of nosocomial infections [14]. However, S. aureus is readily killed with BACs because their cell walls are chiefly composed of slightly-negatively-charged peptidoglycan and techoic acids [10,18]. These surface structures also lack effective permeability properties and allow uptake of more BACs and other antimicrobial substances [10,18].
Enveloped viruses
Some viruses require a lipid envelope, which serves a dual function: first, as a protective barrier from harsh environmental conditions of pH or desiccation, and secondly as an undesirable target for BAC-based antiseptics [18]. Therefore, enveloped viruses like human immunodeficiency virus (HIV), hepatitis B virus (HBV), influenza virus, measles virus, vaccinia virus, meningopneumonitis virus, Semliki Forest virus, canine distemper virus, rabies virus, fowl laryngotracheitis, and feline pneumonitis virus are all susceptible to BACs [2,18,20].
General Antiseptic Resistance
Currently suggested antiseptic resistance mechanisms resemble those conferring antibiotic resistance: enzymatic degradation, biofilm formations, active efflux, and impermeability [9,19,23]. Generally, these mechanisms may originate as either an intrinsic (natural) or acquired property (by genetic mutation or horizontal gene transfer) of the microorganism [18]. Additional studies found that genes encoding resistance components located on mobile genetic elements (eg. plasmids or transposons) may be transferred from one microorganism to another, as is the case with Staphylococci carrying QAC-resistant plasmids encoding efflux pumps [3,7,20]. Therefore in response to antiseptic action, different microorganisms exhibit these aforementioned intrinsic and acquired antiseptic resistance mechanisms.
Impermeability of Benzalkonium Chloride
In particular, impermeability by additional or architecturally-complex surface layers in addition to the cellular membrane are commonly exhibited by a variety of microorganisms that render BAC less efficacious [16,18]. Not only is BAC prevented from causing cell lysis, but the uptake of BAC and other active agents in hand sanitizers is severely inhibited [18]. Therefore, even at high concentrations, BACs tend to be “non-cidal” and are rather bacteriostatic and mycobacteriostatic [21].
Bacteriostatic
Pseudomonas aeruginosa, a Gram-negative bacterium, is a common opportunist pathogen causing respiratory tract infections like pneumonia [5,6,18]. Generally, Gram-negative bacteria like P. aeruginosa are less permeable to QACs than non-sporulating, non-mycobacterial Gram-positive organisms [6,18]. The difference in uptake regulation by Gram-negative bacteria is mediated by structural differences in the outer membrane, porins, and efflux pumps [19,23]. While inner membranes have a phospholipid bilayer, the outer leaflet of an outer membrane is composed of lipopolysaccharides (LPS) [24]. The outer membrane’s reduced permeability is a result of strong LPS-LPS lateral interactions, where a highly anionic “R-core” region on the LPS links interacts with soluble cations to link LPS molecules together [9,14,23]. The outer membrane’s hydrophobic core has greater London dispersion forces since LPS has more hydrocarbon tails per molecule than a phospholipid molecule [19]. Small porins studding this outer membrane facilitates diffusion of hydrophilic solutes, but can strictly restrict larger molecules of BAC [14,23]. Although P. aeruginosa is a model organism of BAC impermeability, other species have been reported as susceptible to BAC [17].
Mycobacteriostatic
Mycobacteria are some of the most antiseptic-resistant opportunistic pathogens, characterized by highly hydrophobic cell walls of a complex mycoyl arabinogalactan-peptidoglycan structure [11,16]. Experiments involving cell wall synthesis inhibitors suggests that a cell wall component like lipid content is responsible for antiseptic resistance [23]. Further studies have shown Mycobacterium tuberculosis (high lipid content) to be more resistant to BAC than M. phlei (lower lipid content), which supports the idea of an increased lipid content correlating to increased BAC impermeability [18,23].
References
1.Aiello, A.E., Coulborn, R.M., Perez, V., and Larson, E.L. 2008. Effect of hand hygiene on infectious disease risk in the community setting. American Journal of Public Health, 98 (8): 1372-1381.
2.Armstrong, J.A., and Froelich, E.J. 1964. Inactivation of viruses by benzalkonium chloride. Applied Microbiology, 12 (2): 132-137.
3.Bjorland, J., Steinum, T., Kvitle, B., Waage, S., Sunde, M., and Heir, E. 2005. Widespread distribution of disinfectant resistance genes among Staphylococci of bovine and caprine origin in Norway. Journal of Clinical Microbiology, 43 (9): 4363-4369.
4.Campanac, C., Pineau, L., Payard, A., Baziard-Mouysset, G., and Roques, C. 2002. Interactions between biocide cationic agents and bacterial biofilms. Antimicrobial agents and chemotherapy, 46 (5): 1469-1474.
5.Chi, S.Y., Kim, T.O., Park, C.W., Yu, J.Y., Lee, B., Lee, H.S., Kim, Y.I., Lim, S.C., and Kwon, Y.S. 2012. Bacterial pathogens of ventilator associated pneumonia in a tertiary referral hospital. Tuberculosis and Respiratory Diseases, 73 (1): 32-37.
6.Chuanchuen, R., Beinlich, K., Hoang, T.T., Becher, A., Karkhoff-Schweizer, R.R., and Schweizer, H.P. 2001. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrobial Agents and Chemotherapy, 45 (2): 428-432.
7.Ciric, L., Mullany, P., and Roberts, A.P. 2011. Antibiotic and antiseptic resistance genes are linked on a novel mobile genetic element: Tn6087. Journal of Antimicrobial Chemotherapy, 66: 2235-2239.
8.Cook, H.A., Cimiotti, J.P., Phyllis D.L., Saiman, L., and Larson, E.L. 2007. Antimicrobial resistance patterns of colonizing flora on nurses’ hands in the neonatal intensive care unit. American Journal of Infection Control, 35 (4): 231-236.
9.Coughlin, R.T., Tonsager, S., and McGroarty, E.J. 1983. Quantitation of metal cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry, 22 (8): 2002-2007.
10.Fazlara, A., and Ekhtelat, M. 2012. The disinfectant effects of benzalkonium chloride on some important foodborne pathogens. American-Eurasian Journal of Agricultural & Environmental Science, 12 (1): 23-29.
11.Frenzel, E., Schmidt, S., Niederweis, M., and Steinhauer, K. 2011. Importance of porins for biocide efficacy against Mycobacterium smegmatis. Applied and Environmental Microbiology, 77 (9): 3068-3073.
12.Gordin, F.M., Schultz, M.E., Huber, R.A., and Gill, J.A. 2005. Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub. Infection control and hospital epidemiology, 26 (7): 650-653.
13.Hammond, B., Ali, Y., Fendler, E., Dolan, M,D.S. 2000. Effect of hand sanitizer use on elementary school absenteeism. American Journal of Infection Control, 28:340–6.
14.Kampf, G., and Kramer, A. 2004. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17 (4): 863-893.
15.Lau, C.H., Springston, E.E. , Sohn, M.W., Mason, I., Gadola, E., Damitza, M., and Gupta, R.S. 2012. Hand hygiene instruction decreases illness-related absenteeism in elementary schools: a prospective cohort study. Biomedical Central Pediatrics, 12 (52): 1-7.
16.Leaper, D., McBainm, A.J., Kramer, A., Assdian, O., Sanchez, J.L.A., Lumio, J, and Kiernan, M. 2010. Healthcare associated infection: novel strategies and antimicrobial implants to prevent surgical site infection. Annals of the Royal College of Surgeons of England, 92: 452-458.
17.Mangalappalli-Illathu, A.K. , and Korber, D.R. 2006. Adaptive resistance and differential protein expression on Salmonella enteric serovar enteritidis biofilms exposed to benzalkonium chloride. Antimicrobial agents and chemotherapy, 50 (11): 3588-3596.
18.McDonnell, G., and Russell, A.D. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clinical Microbiology Reviews, 12 (1): 147-179.
19.Nic, M., Jirat, B., and Kosata, B. 2006. IUPAC of quaternary ammonium compounds [online]. Available from http://goldbook.iupac.org/Q05003.html [accessed 26 October 2012].
20.Oxford, J.S., Potter, W., McLaren, C., and Hardy, W. 1971. Inactivation of influenza and other viruses by a mixture of virucidal compounds. American Society for Microbiology, 21 (4): 606-610.
21.Prazuck, T., Compte-Nguyen, G., Pelat, C., Sunder, S., and Blanchon, T. 2010. Reducing gastroenteritis occurrences and their consequences in elementary schools with alcohol-based hand sanitizers. The Pediatric Infectious Disease Journal, 29 (11): 994-998. 22.Reynolds, S.A., Levy, F., and Walker, E.S. 2006. Hand sanitizer alert. Emerging Infectious Diseases, 12 (3): 527-529.
23.Sheldon, A.T. 2005. Antiseptic “resistance”: real or perceived threat? Clinical Infectious Diseases, 40 (11): 1650-1656.
24.To, M.S., Favrin, S., Romanova, N., and Griffiths, M.W. 2002. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Applied and Environmental Microbiology, 68 (11): 5258-5264.
25.White, C., Kolble, R., Carlson, R., Lipson, N., Dolan, M., Ali, Y., and Cline, M. 2003. The effect of hand hygiene on illness rate among students in university residence halls. American Journal of Infection Control, 31:364–370.