Fecal Microbiota Transplantation: A Potential Treatment for Crohn’s Disease
The human body has been proposed as a “superorganism” that includes bacterial and human genes (Ley 2006) where the number of microbes are as many as 10-fold cells and microbe genes are 100-fold that of human. Gut microbial cells have been associated with host physiology and immune homeostasis. It has been proposed that changes in the composition of microbial population, a term called dysbiosis [4], is related to gastrointestinal diseases such as ulcerative colitis and Crohn’s disease[5].
Crohn's disease (CD) is a relapsing inflammatory disease that is associated with autoimmune disorders. It affects the gastrointestinal tract and symptoms often include abdominal pain, diarrhea of mucus or blood, fever, and weight loss [6]. There is currently no available treatment to cure this chronic illness, therefore long-term treatment is often used even though there are concerns [7] regarding long-term use of various drugs prescribed to manage CD [8]. When long-term CD treatment has been exhausted, fecal microbiota transplantation may be a promising rescue therapy.
Fecal microbiota transplantation (FMT)[9] is a procedure in which fecal matter is collected from a healthy donor, mixed with a saline or other solution, strained, and placed in a patient via colonoscopy, endoscopy, sigmoidoscopy, or enema. Prior to 2013, research that explored the treatment efficacy of FMT was generally limited to patients with Clostridium difficile infections (CDI) but over the last few years, FMT has increasingly become a popular inquiry among patients with inflammatory bowel diseases (IBD) other than CDI. [10]
Current Commonly-used Medications to Treat CD
A person's therapeutic needs may change over time depending on the progress of the disease while the treatment is always geared toward achieving remission, maintaining remission or improving quality of life for the patient. Medications may be given orally, intravenously, subcutaneously, or topically. Medications for CD fall into five basic categories: aminosalicylates,corticosteroids, immunomodulators, and antibiotics . [11]
Aminosalicylates
Aminosalicylates are typically given orally or applied rectally, they decrease inflammation at the inner wall of the intestine and are effective in treating relapses and mild-moderate episodes of IBD. Examples of Aminosalicylates includes sulfasalazine, balsalazine, melamine, and olsalazine. Some common side effects of aminosalicylates include headache, nausea, fever, rash, and reversible infertility in men.
Corticosteroids
Corticosteroids, given orally, intravenously or rectally, suppress the immune system and decreases the body's ability to maintain an inflammatory process. Examples of corticosteroids are prednisone, prednisolone, methylprednisolone, and budesonid. Pred- nisone and prednisolone are used for people with moderate-to-severe Crohn’s disease and ulcerative colitis. Budesonide is used for people with mild-moderate ileal Crohn’s disease, and right-sided colon Crohn’s disease. Corticosteroids are only effective for short-term acute episodes and are not recommended for long-term use due to various side effects of the drugs. [12]
Immunomodulators
Usually given orally, this class of medication modifies the body’s immune system so that it cannot cause ongoing inflammation. Immunomodulators are typically used in people for whom aminosalicylates and corticosteroids haven’t been effective, or have been only par-tially effective. They may be useful in reducing or eliminating reliance on corticosteroids. They also may be effective in maintaining remission in people who have not responded to other medications but may take up to three months to effects of the medication on the patient.
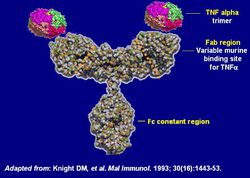
Biologic therapies
Biologic therapies are used for people of moderate to severe Crohn’s, biologics is often used as a strategy to reduce steroid use. These therapies are bio-engineered drugs that target very specific molecules involved in the inflammatory process. These are not drugs but proteins that target the action of certain other proteins that cause inflammation. This class of proteins includes adalimumab, certolizumab pegol, golimumab, infliximab, and natalizumab.
[[Image:FInf[13] ]]
Antibiotics
Antibiotics are antimicrobials used to treat bacterial infections. Infections of the stomach lining can be symptoms of Crohn’s disease and other IBD and are often treated with antibiotics.
Eastern use of FMT
Origin of FMT
FMT has been a treatment in traditional Chinese medicine since as early as the 4th century. Hou Bei Ji Fang” (meaning “Handy Therapy for Emergencies”), the first Chinese handbook of emergency medicine, accounted for the success of the first FMT cases by famous traditional Chinese medicine doctor Ge Hong. He lived during the Dong-jin Dynasty and used human fecal suspension to treat patients who had food poisoning or severe diarrhea (Zhang et al. 2012). The second record of FMT being used to treat physical illnesses was from the 16th century Ming Dynasty. In the most famous traditional Chinese medicine book “Ben Cao Gang Mu ” (meaning “Compendium of Materia Medica”), Li Shizhen, another well-known eastern medicine doctor, described a series of prescriptions using fermented fecal solution, fresh fecal suspension, dry feces, or infant feces for effective treatment of abdominal diseases with severe diarrhea, fever, pain, vomiting, and constipation. Treatments containing fecal matter were labeled as “yellow soup” and other names for aesthetics. [14]
Methods of the First Standardized FMTs in China
Donor Screening
Patients can self-identify potential donor or the doctor can recommend fecal microbiota from the bacterial bank. The volunteers who had passed the selection criteria are given a laxative before defecation. Criteria for screening include: history of drug use where the donor must not have received antibiotics, laxative or diet pills, received immunomodulators[15] or chemotherapy within the last 3 months of the donations; history of diseases where infectious diseases such as diabetes, obesity, chronic diarrhea, constipation, colorectal, IBS, IBD, colorectal polyps, caner, allergy, metabolic syndrome and chronic fatigue syndrome would disqualify a person from donating; and stool testing for parasites. [16]
Patient preparation
Conventional treatment of CD (steroid,immunomodulator, biotherapy and traditional Chinese medicine) is stopped one week prior to FMT. Disease duration, disease localization, disease behavior, treatment and surgical history, body weight, and Harvey–Bradshaw Index (HBI), an index created by calculating patient's physical status using a defined scoreboard, are assessed. Peripheral blood is collected for chemical and biological analyses. Daily doses of 3g mesalazine[17] is given to patient.
FMT procedure
1. Collect feces with a sterilized container and transfer feces to a blender
2. Prepare fecal suspension by adding 0.5-1L of 0.9% saline to the blender and blender mixture to suspension
3. Filter using a micro-strainer four times and collect the suspension
4. Centrifuge for 3 minutes
5. Discard the supernatant and ass 50 ml of saline and centrifuge again
6. Centrifuge and wash step is repeated 3-5 times
7. Discard supernatant, leaving crudely purified fecal microbiota
8. Dilute the flora with 1.5 fold 0.9% normal saline and mix the mixture
9. Fresh, concentrated fecal microbiota suspension is to be administered to the intestine immediately
10. Flora can be stored with 10% sterile glycerol at -80°C
11. For endoscopic procedure, 150-200 mL liquid suspension of approximately 1 part cal flora and 2 parts normal saline is transplanted into patient;s mid-gut through table in the gastroscope under anesthesia
[18]
Chinese Case Study: The first FMT for severe enterocolonic CD
A 32-year-old Chinese man developed severe enterocolonic CD where he was hospitalized in 2012 for progressive abdominal pain, bloody and purulent diarrhea and high fever of 38 °C-39.5 °C. He was diagnosed in 2010 and managed his disease through daily intravenous medicines. A CT Scan showed an abdominal mass of size 14 cm × 8 cm × 10 cm. His sigmoid colon was severely inflamed and he was given intravenous antiobiotics for 10 days but frequently experienced fever and abdominal pain; plus, the abdominal mass size was unchanged. After the patient expressed interest in a clinical trial, various tests were conducted and the donor found was his 10-year-old, healthy daughter. A week after FMT, his previous symptoms were dramatically alleviated and the of inflamed mass was reduced. Additionally, the Crohn's Disease Activity Index (CDAI) score was reduced to 228. He had a severe cold in the whole third week after he was discharged with clinical improvement. At one month of follow-up after FMT, his CDAI score was further reduced to 143, which met the criteria of clinical remission. To that effect, three months after FMT, the CDAI score was further reduced to 62, suggesting sustained clinical remission. His appetite improved, album total cholesterol increased to 47.5 g/L (within normal range of 35-55) and he gained 11 kg in nine months.[19]
Western Emergence of FMT
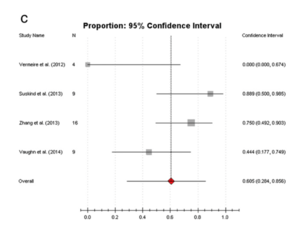
Using 16 S rRNA gene-based Single-strand_conformation_polymorphism single strand conformation polymorphism (SSCP), it was discovered that patients suffering from IBD has less diversity than healthy individuals[20]. Although studies have revealed that IBD is associated with abnormal gut microbiota composition, it is difficult to identify a general disease-associated microbiota pattern due to host-specific composition gut microbiota [21]. In a review summarizing 12 reports including 111 patients with IBD who received FMT therapy, it was found that the overall success rate of FMT in adults was 77.8%[22]. Interestingly, another review combining 18 reports of 39 CD patients through a different screening process reported an FMT efficacy on CD to be 60.5%[23]. Although the practice of FMT has been further explored only in recent years and no standardized procedure has been dictated, its high efficacy couple with lower side effects signify that this procedure can be a promising treatment for CD and Crohn’s Disease].
Safety of FMT and endoscopic procedure
In a case study following 49 patients who received FMT through the mid-gut, there was no severe or obvious adverse events during endoscopic infusion after FMT and long-term follow-up of 6-15 months. [24]
FMT-Related Factors
Genetic background or close contact with recipients did not significantly affect the outcome of patients’ clinical response at six months after FMT. The efficacy of using fresh fecal microbiota seemed higher than that using frozen fecal microbiota, but the difference on the clinical improvement or clinical remission was not significant. There were no differences in
patients’ clinical response between the two age groups of donors over 14 and under 14 years of age. [25]
Further Reading and Viewing
While the idea of using FMT to treat Crohn's disease has been around since 1998, the U.S. has still yet to develop an official protocol to treat Crohn's disease using FMT. It is said that Crohn's disease could be a result of genetics as well as the host environment where something we are currently unsure of causes a shift in the human gut micro biome. A popular speculation links Crohn's Disease to Mycobacterian Avium Paratuberculosis (MAP), a bacteria that causes a cow version of the inflammatory bowel syndrome called Johne's Disease. Some people in the community proposes that MAP can be developed into a vaccine to prevent Crohn's Disease.
Crohn's MAP Vaccine— Professor John sermon-Taylor and : MAP Infrequent Human Pathogen or Public Health Threat —A Report from the American Academy of Microbiology
References
[1] Baumgart DC and Sandborn WJ. (2012). Crohn's disease. The Lancet. 380 (9853): 1590–605.
Edited by Phuongngan Bui, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.
