Formation of Intrauterine Adhesions from the Disruption of the Vaginal Microbiome
Introduction
Gardnerella vaginalis is a rod-shaped, gram-variable bacteria that are normally found in small quantities within the larger, diverse vaginal microbiome (Figure 1). [1] A certain amount of these bacteria in the vagina is considered safe, but an increase could lead to bacterial vaginosis, a painful condition that is caused by the change of dominant vaginal bacteria shifting from a form of Lactobacillus to a range of diverse, mostly anaerobic bacteria like Gardnerella vaginalis, Prevotella bivia, Bacteroides fragilis, and other species of the fragilis group. [2] [3]
Interestingly, these bacteria, despite being normally found in the vagina in small quantities, can cause severe, lasting damage in the uterus in the form of intrauterine adhesions. If bacterial vaginosis is not properly treated, the vaginal microbiome will be unable to protect against harmful bacteriasuch as Gardnerella vaginalis from entering into the uterus, allowing it to degrade the endometrium and cause adhesions. Due to their scar-like nature, these uterine adhesions are normally characteristic of Asherman’s syndrome, a chronic disease that causes scar tissue formation upon the endometrium or the tissue that lines the uterus due to various forms of trauma (Figure 2). [4] [5] [6] The trauma that contributes to Asherman's syndrome can also be derived from one's genetics, surgical procedures or treatments, past abortions, pregnancy, and severe infection. [7] [8] [9]

These intrauterine adhesions can cause pelvic pain and an increased risk of endometrial cancer, as they do not allow for the proper building or shedding of the endometrium throughout a menstrual period.
[8]
One’s composition of their vaginal microbiome has a role in their susceptibility to having Gardnerella vaginalis overtake their microbiome and enter their uterus to cause these intrauterine adhesions, even if they are not genetically predisposed to Asherman’s syndrome.
[10]
[6]
[7]
The defined categories for the various, dominant bacteria in one’s microbiome that have play a large role in one’s immune response to foreign bacteria or an imbalance in their vaginal microbiome are called community state types (CSTs).
[11]
CSTs have been proven to be highly influenced by one’s genetics and race, leading some populations to be more susceptible to not only bacterial vaginosis but the possible ensuing uterine damage. Other reproductive diseases such as PCOS and endometriosis have been accredited as having hereditary influence. [12]
Interestingly, PCOS patients are known to have increased levels of Gardnerella vaginalis,
so patients that are susceptible to those bacteria have higher risks of experiencing the effects of two major reproductive disorders. The derivation of intrauterine adhesions by Gardnerella vaginalis can lead to a misdiagnosis of either endometriosis or endometritis.

Microbial Impact
When essential vaginal bacteria, such as Lactobacillus, are not present at regular levels, the pH balance of the vagina can rise, which allows for bacteria like Gardnerella to overtake the vaginal microbiome. These bacteria are only able to damage the endometrium when the ability to enter the uterus finally arises, normally due to factors such as recent sexual activity, phases of the menstrual cycle, etc.
[13]
When bacteria such as Gardnerella enter the uterus, they degrade the endometrium’s epithelial glycocalyx, which is the sugar layer that lies on the outermost layer of the uterine lining and does not shed during a menstrual period. These bacteria adhere to the uterine lining and create sialidase, which causes inflammation and damage to the vaginal epithelial cells by removing sialic acid residues. By removing these negatively charged sugars, they firstly hinder cellular communication which then leads to the general, innate immune response that will create these adhesions or scar-like tissue on the endometrium.
[14]
Genetic Influence
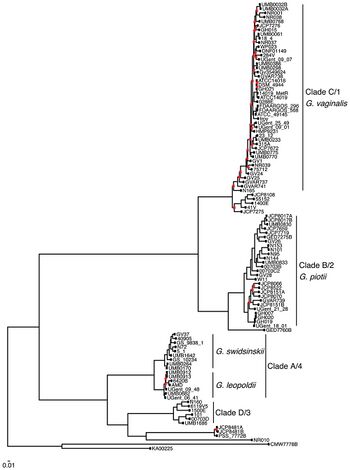
Gardnerella vaginalis, and its related strains continue to evolve, possibly due to their large accessory genomes. The implications of this are vast, with some populations already at an increased risk of contracting Gardnerella vaginalis and the precondition of bacterial vaginosis due to their given genetics and environment [15] [16]
The evolution of these bacteria could only increase the risk of those already susceptible to a disrupted vaginal microbiome due to their genetics or environment, possibly leading to infertility and lower birth rates in certain populations.
[17]
Despite their pathogenic ability, Gardnerella vaginalis is commonly found in healthy asymptomatic individuals. But, various ecotypes of these bacteria, such as G. piotii, G. swidsinskii, G. leopoldii, and another similar bacteria named Prevotella bivia have also been found in stable, vaginal microbiomes.
[18]
[19]
Over time,Gardnerella vaginalis has evolved, like many other bacteria, to combat the responsive human immune system or the Lactobacillus bacteria that inhibits its ability to grow in large quantities within the vagina.
Gardnerella vaginalis appears to be the common ancestor of the new strains of Gardnerella, for its accessory genome is large compared to its relatives such as Bifidobacterium.
[15]
This large accessory genome could have been the source of convergence/speciation with possible mutations coding for new functions involved in drug/antibiotic resistance, metabolism, and virulence (Figure 3).
[15]
Of the classified ecotypes of Gardnerella, none are known to be crucial for the overall health of the vaginal microbiome. Some forms of Gardnerella, like those in clade 1 and 2, display a greater association with bacterial vaginosis and ensuing, more adverse health effects such as intrauterine adhesions, painful menstrual periods, or infertility, as they are less susceptible to the protective nature of Lactobacillus.
[15]
[20]
[21]
The other way Gardnerella and similar bacteria, especially Prevotella , are influenced by genetics is through the host’s genome.
[22] Every vaginal microbiome is characterized by its community state type, or the relative amounts of and the dominant form of Lactobacillus bacteria.
[23]
Every strain of Lactobacillus bacteria takes up space on the vaginal wall, produces bacteriocins, and decreases inflammation in the genetic tract.
[11]
Each form of Lactobacillus produces a different amount or kind of lactic acid, like with L. coryniformis producing lactic acid in D form and most of the other strains producing the L form of lactic acid that supports the regulation of the vaginal pH.
[24]
A characteristic of vaginal dysbiosis is either a high or low amount of lactic acid, depending on what causes the vaginal pH to deviate from its typical standard of around 3.8-4.5.
[11]
Any significant deviation from the normal vaginal pH increases the risk of infection and bacterial vaginosis, for it decreases the protection that the stable vaginal microbiome produces.
[23]
But, lower amounts of lactic acid, even normally, especially increase the likelihood of contracting bacterial vaginosis, for Gardnerella can live in higher amounts
[25]
Community state types 4A and 4B, for example, normally produce low amounts of lactic acid because there is no form of dominant Lactobacillus that will contribute in creating a higher vaginal pH or protecting the vaginal canal, allowing Gardnerella to grow.
[25]
Epigenetic Impact
The field of epigenetics, while it is largely undiscovered, the implications and current findings in the field are significant. As our world changes around us, it also continues to change inside of us. As our environment changes from climate change and increasing levels of air pollution, it is already impacting the lives of those who will come after us: our children. A recent experiment focused on the impact of the most abundant air pollutants (PM2.5, PM10, O3, NO2, CO, and SO2) on ovarian response. Ovarian response is defined as the actual oocyte yield after ovarian stimulation. [26] [27] This experiment found that after short periods of exposure to these pollutants, ovarian response significantly decreased. [26]
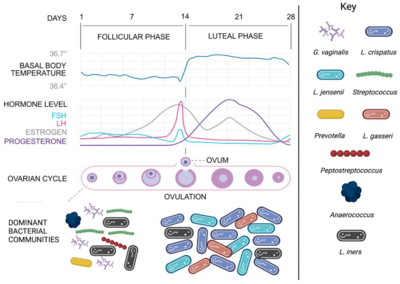
A decreased ovarian response can lead to the gradual decrease of ovulation, which would limit the ability to allow for fertilization. Ovarian response largely contributes to the menstrual cycle as a whole, for menses will only occur if ovulation did not result with a fertilized zygote. Also, the stimulation of hormone production by ovulation and the related eggs and follicles regulates the period of menses. During ovulation, the vaginal microbiome remains relatively stable, with a low pH and a large Lactobacillus population.
[28]
But, during menstruation, the vaginal microbiome has been found to be at its highest level of diversity, due to the rise in vaginal pH and iron levels.
[29]
This increase in diversity is possibly due to the decrease of Lactobacillus bacteria, which would allow for bacteria such as Gardnerella and other anaerobic bacteria to become dominant. Also, the presence of additional nutrients in the vagina during menses could also allow for the increased diversity (Figure 4). Menorrhea contains high levels of glycogen, a sugar molecule that is produced by the lining of the vaginal epithelium, and proteins, which can be used by harmful bacteria such as Gardnerella vaginalis and its related counterparts.
[30]
[31]
The correlation between environmental changes and risk for bacterial vaginosis or the entrance of Gardnerella vaginalis into the uterus could inherently decrease fertility on a large scale.
Also, the persistent contraction of bacterial vaginosis or dysbiotic levels of Gardnerella vaginalis has been attributed to early-life sexual abuse, chronic discrimination, and the lack of parental homeownership. Due to these stressors, the expression of certain genes for reproductive system function is decreased to save energy, leading to the dysbiosis of the vaginal microbiome.
[32]
So, while genes coding for standard phenotypes can impact one's fertility or susceptibility to Gardnerella vaginalis, their lived experiences can either put them at risk or at an increased risk, leading to possible generational struggles with infertility.
Conclusion
Many factors contribute to the state of one’s reproductive health, with it requiring a genetically predetermined balance that must be maintained. The lack of education and focus on the subject fosters a sense of unfamiliarity with one’s own body, leading to a largely shared internal shame and health anxiety. The composition of the vaginal microbiome varies by individual, which complicates the analysis and diagnosis of not only the problems, but the preconditions that could be monitored and prevented. The variety of factors that can negatively impact vaginal health are vast to an unknown extent. Both the genetic and responsive ability of the female genital system speaks to its fascinating complexity. While the immune responses that the female reproductive system uses are not always the most productive or have the least symptoms, its ability to respond to a growing variety of threats or harmful microbes demonstrates its extensive ability to protect the system and its crucial functions.
References
Edited by Mariyah Rumpca-Veronese, student of Joan Slonczewski for BIOL 116, 2024, Kenyon College.
- ↑ Marín E, Haesaert A, Padilla L, Adán J, Hernáez ML, Monteoliva L, et al. Unraveling Gardnerella vaginalis Surface Proteins Using Cell Shaving Proteomics. Frontiers in Microbiology. 2018 May 15;9. https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2018.00975/full
- ↑ Bergan T. Anaerobic bacteria as cause of infections in female genital organs. Scandinavian Journal of Gastroenterology Supplement [Internet]. 1983 [cited 2023 Oct 29];85:37–47. https://pubmed.ncbi.nlm.nih.gov/6353553/
- ↑ Anton L, Ferguson B, Friedman ES, Gerson KD, Brown AG, Elovitz MA. Gardnerella vaginalis alters cervicovaginal epithelial cell function through microbe-specific immune responses. Microbiome. 2022;10(1). https://doi.org/10.1186/s40168-022-01317-9
- ↑ Wang J, Li Z, Ma X, et al. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nature Communications. 2021;12(1):4191. https://doi.org/10.1038/s41467-021-24516-8
- ↑ Dun S, Liu C, Li N. Changes of Vaginal Microecology of Women with Intrauterine Adhesions. International Journal of Women's Health. 2023;Volume 15:857-867.https://doi.org/10.2147/ijwh.s407010
- ↑ 6.0 6.1 Smikle C, Shailesh Khetarpal. Asherman Syndrome [Internet]. Nih.gov. StatPearls Publishing; 2019 https://www.ncbi.nlm.nih.gov/books/NBK448088/
- ↑ 7.0 7.1 Wang J, Li Z, Ma X, et al. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nature Communications. 2021;12(1):4191. https://doi.org/10.1038/s41467-021-24516-8
- ↑ 8.0 8.1 Smikle C, Shailesh Khetarpal. Asherman Syndrome [Internet]. Nih.gov. StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK448088/
- ↑ Dun S, Liu C, Li N. Changes of Vaginal Microecology of Women with Intrauterine Adhesions. International Journal of Women's Health. 2023;Volume 15:857-867. https://doi.org/10.2147/ijwh.s407010
- ↑ Santamaria X. Asherman’s Syndrome: it may not be all our fault [Internet]. Oup.com. 2024 [cited 2024 Dec 5] https://academic.oup.com/humrep/article/33/8/1374/5061901
- ↑ 11.0 11.1 11.2 Maduta CS, McCormick JK, Dufresne K. Vaginal community state types (CSTs) alter environmental cues and production of the Staphylococcus aureus toxic shock syndrome toxin-1 (TSST-1). Journal of Bacteriology [Internet]. 2024 Mar 21. https://pmc.ncbi.nlm.nih.gov/articles/PMC10955855/
- ↑ Bischoff F, Simpson JL. Genetic Basis of Endometriosis. Annals of the New York Academy of Sciences. 2004;1034(1):284-299. Accessed December 5, 2024. https://doi.org/10.1196/annals.1335.030
- ↑ About Bacterial Vaginosis (BV) 2024 [cited 2024 May 23] https://www.cdc.gov/bacterial-vaginosis/about/index.html#:~:text=However%2C%20we%20do%20know%20the,your%20risk%20for%20getting%20BV.CDC.
- ↑ Segui-Perez C, Jongh R de, Robin, et al. Prevotella timonensis degrades the vaginal epithelial glycocalyx through high fucosidase and sialidase activities. mBio. 2024;15(9).https://doi.org/10.1128/mbio.00691-24
- ↑ 15.0 15.1 15.2 15.3 Cornejo OE, Hickey RJ, Suzuki H, Forney LJ. Focusing the diversity of Gardnerella vaginalis through the lens of ecotypes. Evolutionary Applications. 2017;11(3):312-324. https://doi.org/10.1111/eva.12555
- ↑ Murphy K, Mitchell CM. The Interplay of Host Immunity, Environment and the Risk of Bacterial Vaginosis and Associated Reproductive Health Outcomes. Journal of Infectious Diseases. 2016;214(suppl 1):S29-S35. https://doi.org/10.1093/infdis/jiw140
- ↑ Alcendor DJ. Evaluation of Health Disparity in Bacterial Vaginosis and the Implications for HIV-1 Acquisition in African American Women. American Journal of Reproductive Immunology. 2016;76(2):99-107. https://doi.org/10.1111/aji.12497
- ↑ Bohr LL, Mortimer TD, Pepperell CS. Lateral Gene Transfer Shapes Diversity of Gardnerella spp. Frontiers in Cellular and Infection Microbiology. 2020;10. https://doi.org/10.3389/fcimb.2020.00293
- ↑ George SD, Van Gerwen OT, Dong C, et al. The Role of Prevotella Species in Female Genital Tract Infections. Pathogens. 2024;13(5):364. https://doi.org/10.3390/pathogens13050364
- ↑ Shvartsman E, Hill JE, Sandstrom P, MacDonald KS. Gardnerella Revisited: Species Heterogeneity, Virulence Factors, Mucosal Immune Responses, and Contributions to Bacterial Vaginosis. Infection and Immunity. 2023;91(5). https://doi.org/10.1128/iai.00390-22
- ↑ Qin H, Xiao B. Research Progress on the Correlation Between Gardnerella Typing and Bacterial Vaginosis. Frontiers in Cellular and Infection Microbiology. 2022;12. https://doi.org/10.3389/fcimb.2022.858155
- ↑ Mehta SD, Nannini DR, Otieno F, et al. Host Genetic Factors Associated with Vaginal Microbiome Composition in Kenyan Women. Fodor A, ed. mSystems. 2020;5(4).https://doi.org/10.1128/msystems.00502-20
- ↑ 23.0 23.1 De Seta F, Campisciano G, Zanotta N, Ricci G, Comar M. The Vaginal Community State Types Microbiome-Immune Network as Key Factor for Bacterial Vaginosis and Aerobic Vaginitis. Frontiers in Microbiology. 2019;10. https://doi.org/10.3389/fmicb.2019.02451
- ↑ Ibrahim SA. Lactobacillus - an overview | ScienceDirect Topics [Internet]. www.sciencedirect.com. 2016. Available from: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/lactobacillus
- ↑ 25.0 25.1 Patterson JL, Girerd PH, Karjane NW, Jefferson KK. Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. American Journal of Obstetrics and Gynecology. 2007;197(2):170.e1-170.e7. https://doi.org/10.1016/j.ajog.2007.02.027
- ↑ 26.0 26.1 Wu S, Hao G, Zhang Y, Chen X, Ren H, Fan Y, et al. Poor ovarian response is associated with air pollutants: A multicentre study in China. eBioMedicine. 2022 Jul;81:104084. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(22)00265-1/fulltext
- ↑ American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion (2020) [Internet]. www.asrm.org. 2020. https://www.asrm.org/practice-guidance/practice-committee-documents/testing-and-interpreting-measures-of-ovarian-reserve-a-committee-opinion-2020/#:~:text=The%20tests%20discussed%20above%20reflect,oocyte%20yield%20after%20ovarian%20stimulation.
- ↑ Miller EA, Livermore JA, Alberts SC, Tung J, Archie EA. Ovarian cycling and reproductive state shape the vaginal microbiota in wild baboons. Microbiome [Internet]. 2017 Jan 19 [cited 2019 Sep 15];5(1). https://pmc.ncbi.nlm.nih.gov/articles/PMC5248513/
- ↑ Krog MC, Hugerth LW, Fransson E, Bashir Z, Nyboe Andersen A, Edfeldt G, et al. The healthy female microbiome across body sites: effect of hormonal contraceptives and the menstrual cycle. Human Reproduction. 2022 May 11;37(7):1525–43. https://pmc.ncbi.nlm.nih.gov/articles/PMC9247429/#:~:text=During%20the%20menstrual%20phase%2C%20bacterial,(both%20P%20%3C%200.00001).
- ↑ Chaban B, Links MG, Jayaprakash T, Wagner EC, Bourque DK, Lohn Z, et al. Characterization of the vaginal microbiota of healthy Canadian women through the menstrual cycle. Microbiome. 2014;2(1):23. https://link.springer.com/article/10.1186/2049-2618-2-23
- ↑ Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UME, Zhong X, et al. Temporal Dynamics of the Human Vaginal Microbiota. Science Translational Medicine. 2012 May 2;4(132):132ra52–2. https://www.science.org/doi/abs/10.1126/scitranslmed.3003605
- ↑ Cammack AL, Buss C, Entringer S, Hogue CJ, Hobel CJ, Wadhwa PD. The association between early life adversity and bacterial vaginosis during pregnancy. American Journal of Obstetrics and Gynecology. 2011 May;204(5):431.e1–8. https://www.sciencedirect.com/science/article/pii/S0002937811001475
