Fremyella diplosiphon
Classification
Domain: Prokaryota
Kingdom: Eubacteria
Group: Terrabacteria
Phylum: Cyanobacteria
Class: Cyanophyceae
Order: Nostocales
Family: Rivulariaceae
Genus: Microchaete
Species: Diplosiphon
Species
|
NCBI: [1] |
Fremyella diplosiphon
Description and Significance
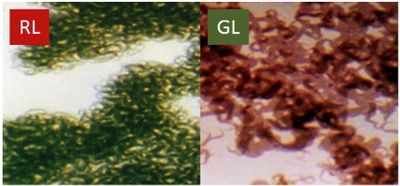
Fremyella diplosiphon is a filamentous chromatically adapting cyanobacterium [1] and was first isolated from a dried pool in Zacapa, Guatemala in 1939 by Paul Standley [2]. Colonies can appear blue-green or red, and cells can appear as thicker brick-shaped filaments or as short and rounded under the microscope, depending on the conditions. It has been studied intensely for the past 40 years for its process known as "proteome remodeling" [10], where the organism adjusts the proteins produced in response to a nutrient limitation (light).
Fremyella diplosiphon is most well known for its complementary chromatic adaptation, which is the ability to adjust photosynthetic receptors in the presence or absence of different colors of light, light intensity, and light quality. Due to this ability, this species has been used to study the biochemical and physiological processes that allow microbes to sense and respond to their environment. Fremyella diplosiphon is capable of adjusting its photosynthetic antennae to harvest different colored light depending on the conditions, making it a key model organism in studying physiological response. Not only is Fremyella diplosiphon an excellent model for phototropism, but response to light differences are also very important in the metabolic process of photosynthesis, which is the necessary anchor of any ecosystem, making Fremyella diplosiphon a key member of its ecological community. Its ecological importance as a photosynthetic Cyanobacterium can also offer insight into the early evolution of ecosystems, as well as the evolution of early life on Earth. The physiological processes that Fremyella diplosiphon possesses could also be utilized as in the development of bioenergy [10].
Genome Structure
Fremyella diplosiphon has one of the largest genomes of bacteria, containing around 9.9 million base pairs in a single circular chromosome. The complete genome sequence can be found at https://www.ncbi.nlm.nih.gov/genome/browse/. Because Fremyella diplosiphon is studied mostly for its proteome remodeling in its physiological response to light, much of the knowledge regarding its genome revolves around the proteins that participate in its light adaptations. One study found that F. diplosiphon's chromosome has a very high resistance to most nucleases, and is therefore able to resist cleavage to its chromosome (8).
Fremyella diplosiphon manipulates the composition of its phycobilisome, the light harvesting organelle, by activating and repressing different operons. The cpeBA operon and the cpeCD operon have been identified as coding for green-light harvesting proteins on the phycobilisome (11). Quantification of the various transcripts produced by F. diplosiphon in both red and green light have shown that, while some transcripts are conserved, many transcripts are expressed very highly in one color light over the other (5). IT has been found that one main light-responsive sensor kinase called RcaE may be responsible for the responses to different light pigments (6). Some genes in F. diplosiphon are found to be conserved across many Cyanobacteria but many of the genes responsible for its complementary chromatic adaptation are specific to this species.
Cell Structure, Metabolism and Life Cycle
Fremyella diplosiphon is a prokaryote that uses oxygenic photosynthesis to capture light energy. The organism’s photosynthetic machinery is packed in the thylakoid membrane at the exterior of the cell and water is used as an electron donor while oxygen is a byproduct. Phycobilisomes attached to the thylakoid membrane function as antennae for the photosystems and the phycobiliproteins within are responsible for Fremyella diplosiphon's blue-green appearance while phycoerythrin is responsible for the red appearance. The light conditions in the organism’s environment determine which phycobiliproteins will accumulate; red phycoerythrin for green light conditions and green phycocyanin for red light conditions. This process of change in protein composition due to environmental stimuli is called complementary chromatic adaptation (CCA) and it allows for more efficient capturing of light energy.
A single cell typically is made up of a thick, gelatinous cell wall without flagella. As a prokaryote, cells of this species do not have nuclei or an internal membrane system. These cells form filaments of varying lengths called hormogonia and often will accumulate on solid surfaces. Hormogonia will typically be more or less cylindrical along their entire length. These multicellular filaments can separate from the main colony by breaking off at a weaker cell called a necridium. The terminal cell of a filament is an elongate, hair like structure called a trichome. It is likely that these trichomes improve the ability of the organism to optimize light exposure.
Reproduction in this species is carried out by asexual, binary fission and the use of hormogonia to create new colonies. These hormogonia separate from trichomes in apical parts. Hormogonia develop heteropolar or firstly isopolar with two central heterocyst, which shift later into the basal position. These heterocyst are the site of nitrogen fixation.
Ecology
Fremyella diplosiphon is a pivotal part of its ecosystem, as it is a photosynthesizing cyanobacteria, and is likely that this species was one of the early Earth lifeforms. F. diplosiphon are found in freshwater aquatic environments and utilize their complementary chromatic adaptation (CCA) to photosynthesize consistently in their aquatic environments that are constantly changing in light intensity and quality. As light wavelengths change greatly throughout the depths of the water column, F. diplosiphon is able to adapt to its environment through CCA and therefore tune its photosynthetic efficiency for the ecosystem.
F. diplosiphon is also a nitrogen and carbon fixing bacterium. The bacteria uses heterocysts (non-growing, oxygen impermeable cells) to protect its nitrogen fixing enzyme nitrogenase. This species of bacteria contributes usable forms of nitrogen and carbon, such as ammonia and organic carbon respectively, into its community. This makes F. diplosiphon a key contributor to both the nitrogen and carbon cycles within its environment.
Pathogenesis
Fremyella diplosiphon is not a known pathogen to plants, animals, or other microbes.
References
1. Cobley, J., & Miranda, R. (1983). "Mutations affecting chromatic adaptation in the cyanobacterium Fremyella diplosiphon." Journal of Bacteriology, 153(3), 1486-1492. Retrieved April 23, 2017.
2. Smithsonian National Museum of Natural History. (n.d.). Retrieved April 23, 2017, from http://collections.nmnh.si.edu/search/botany/?irn=2322547
3. Tamulonis C, Postma M, Kaandorp J (2011) Modeling Filamentous Cyanobacteria Reveals the Advantages of Long and Fast Trichomes for Optimizing Light Exposure. PLoS ONE 6(7): e22084. doi:10.1371/journal.pone.0022084
4. Pattanaik, Bagmi, Melissa J. Whitaker, and Beronda L. Montgomery. "Light quantity affects the regulation of cell shape in Fremyella diplosiphon." Frontiers in Microbiology 3 (2012): 170. Web. 24 Apr. 2017. <http://journal.frontiersin.org/article/10.3389/fmicb.2012.00170 >.
5. Oelmüller, R., Et. al. (1988). Changes in Accumulation and Synthesis of Transcripts Encoding Phycobilisome Components during Acclimation of Fremyella diplosiphon to Different Light Qualities [Abstract]. Plant Physiology, 88(4), 1077-1083. Retrieved April 23, 2017.
6. Pattanaik, B., Whitaker, M. J., & Montgomery, B. L. (2011). Convergence and divergence of the photoregulation of pigmentation and cellular morphology in Fremyella diplosiphon. Plant Signaling & Behavior, 6(12), 2038-2041. doi:10.4161/psb.6.12.18239
7. Shui, J. et al. (2009). Light-Dependent and Light-Independent Protochlorophyllide Oxidoreductases in the Chromatically Adapting Cyanobacterium Fremyella diplosiphon UTEX 481. ;;Plant and Cell Physiology, 50(8), 1507-1521. doi:10.1093/pcp/pcp095
8. Van den Hondel, C.A.M.J.J., van Leen, R.W., van Arkel, G.A., Duyvesteyn, M. and de Waard, A. (1983), Sequence-specific nucleases from the cyanobacterium Fremyella diplosiphon, and a peculiar resistance of its chromosomal DNA towards cleavage by other restriction enzymes. FEMS Microbiology Letters, 16: 7–12. doi:10.1111/j.1574-6968.1983.tb00249.x
9. Montgomery, B. L. (2015). Light-dependent governance of cell shape dimensions in cyanobacteria. Frontiers in Microbiology, 6. doi:10.3389/fmicb.2015.00514
10. Minx, P. (n.d.). Genome: Fremyella diplosiphon. Retrieved April 24, 2017, from http://genome.wustl.edu/genomes/detail/fremyella-diplosiphon/
11. Federspiel, N. A., & Grossman, A. R. (1990). Characterization of the light-regulated operon encoding the phycoerythrin-associated linker proteins from the cyanobacterium Fremyella diplosiphon [Abstract]. Journal of Bacteriology, 172(7), 4072-4081. Retrieved April 23, 2017.
Author
Page authored by Zach Froman and Mollie Carrison, students of Prof. Jay Lennon at Indiana University.