Gonorrhea in Thailand
Introduction
Gonorrhea often flies under the radar because of its’ mild side effects and relatively curable nature; however, in other parts of the world, such as Thailand, gonorrhea has evolved antibiotic resistance to the point where many conventional cures available in the U.S. have no effect. Thailand is a key location for a disease such as gonorrhea because of the blossoming sex industry fueling more and more antibiotic resistance. In addition, gonorrhea can make a person more susceptible to even more deadly conditions such as HIV/AIDS. Understanding the full impact of gonorrhea physically and socially in Thailand will lead to better awareness on how to prevent the disease from spreading.
What is Gonorrhea?
The Disease
Gonorrhea is a common sexually transmitted infection caused by the gram-negative bacterium, Neisseria gonorrhoeae. This disease was first discovered by a German physician named Albert Neisser in 1879 and is most prevalent in men and women between the ages of 15 and 29 (1). The bacterium is very easily transmitted solely between humans through unprotected sex in addition to oral and anal sex; however, using a condom provides almost a 100% prevention of transmission. Mucosal surfaces in the body are prime areas of bacterial infection due to their relative thinness, warm climate and moist surface. These places include the cervix, urethra, uterus and fallopian tubes. The bacteria can also grow in the mouth, throat, anus and even the eyes.
The Microbe
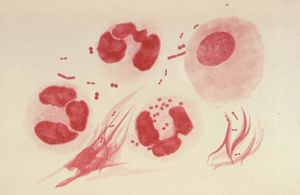
Gonorrhea is caused by the microareophile, Neisseria Gonorrhoeae. N. gonorrhoeae is classified as a gram negative microbe requiring aerobic conditions along with a blood based medium in order grow. A host’s epithelium cells found in the columnar or cuboidal region provide adequate conditions for growth (4). N. gonorrhoeae utilizes transferrin-bound iron for in vitro growth and many strains can also utilize lactoferrin-bound iron. The bacteria can bind only human transferrin and lactoferrin (2). This specificity is the reason these bacteria exclusively infect humans.
In human hosts, N. gonorrhoeae grows best in the reproductive tracts where conditions are moist and warm, making the uterus, cervix, urethra, and the fallopian tubes prime targets for the microbe (6). Upon reaching these cells, N. gonorrhoeae is capable of forming biofilms where their pili function in attaching to the mucosal surface of these sex organs (7). The formation of biofilms makes treating Gonorrhoea difficult since antibiotic resistance arises, which inhibits ingestion and neutrophils (5).
The Ease of Transmission
Transmission of N. gonorrhoeae occurs during unprotected sexual contact where the pathogen may come in contact with warm, moist areas of the vagina, anus, or oral cavities. Ejaculation does not have to take place in order to be infected, simply contact with mucosal membranes. Upon infection there is inflammation in the urethra and cervix caused by N. gonorrhoeae activating inflammatory cytokines and of the pro-IL-1beta-processing complex. As a response to the pathogen, nucleotide-binding domain proteins are synthesized, including NLRP3, activating pyronecrosis (cell death) (8).
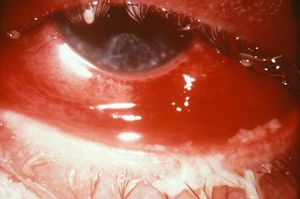
The Symptoms
Many symptoms of a gonorrhea infection are very mild and are characterized by suspicious discharge from the penis or vagina. However, untreated gonorrhea can cause a variety of long-term effects in both men and women. Pelvic inflammatory disease is caused by untreated gonorrhea in women. This disease leads to the creation of pockets of pus to form inside a woman’s pelvis. Not only will this condition cause extreme pelvic pain, it also has the potential to damage a woman’s fallopian tubes and cause infertility. Untreated gonorrhea in men causes a condition known as epididymitis which occurs when ducts attach to the testicles and causes extreme pain and can also cause infertility. Although gonorrhea is a sexually transmitted disease, if left untreated, gonorrhea can spread to other parts of the body forming lesions (9).
The Treatment
Gonorrhea is most commonly treated with antibiotics such as penicillin, tetracycline, spectinomycin, ciprofloxacin, ceftriaxone, cefixime, and azithromycin (3); however, in Asian countries such as Thailand, there have been an increasing number of drug resistant strains of gonorrhea. Today, almost 90% of all gonorrhea strains in Thailand are resistant to penicillin (3). Because gonorrhea has also become resistant to tetracycline and fluoroquinolone antibiotics, there are becoming fewer and fewer drugs that can be taken in order to cure the disease.
Prevention
The first mode of prevention would be to abstain from any sexual contact. If this is not attainable, the next mode of prevention would to be in a monogamous long-term relationship with a tested uninfected individual. Correct latex condom use provides nearly 100% prevention of transmission (9).
Further prevention could be obtained if individual notices symptoms early. If individual is experiencing pain urinating or has an unusual rash, sex should be immediately stopped and the individual should be tested immediately. Following a positive test, the individual should then inform all sexual partners of infection so they too can seek treatment and prevent spreading the disease themselves. During treatment for gonorrhea, these individuals should refrain from sexual contact until treatment is fully complete in order to prevent getting re-infected (9).
Why Gonorrhea in Thailand?
The Problem of Gonorrhea in Thailand
Neisseria gonorrhoeae has become the problem it is today in Thailand for numerous reasons. The most notable issue is Neisseria gonorrhoeae’s persistent resistance to various antibiotics. This complicates its treatment, making control of the disease very difficult. A study in 1980 proved that penicillinase (beta-lactamase)-producing N. gonorrhoeae existed at high levels in clinical isolates in Thailand. Resistance to penicillin treatment was thus established (11). By 1983 tetracycline- resistant, beta-lactamase-producing N. gonorrhoeae proved tetracycline an ineffective treatment. Spectinomycin resistance followed (10). In 2000 the Thai Ministry of Public Health recommended against the use of fluoroquinolones because of high ciprofloxacin-resistant strains (12). Neisseria gonorrhoeae continues to rapidly develop resistance to the antibiotics used to kill the disease making medical care itself very problematic.
The sex industry of Thailand is largely responsible for the high frequency of sexually transmitted disease contraction, gonorrhea being the most common. Due to the rise in tourism to Thailand in the early 90s, a huge sex industry has emerged. It is likely self-medication of sex industry workers is a significant factor in developing antibiotic resistance (10).
Thailand vs. Gonorrhea
In 2001, Thailand became the first third world country to provide universal health coverage. Prior to 2001, the only public health insurance program was the Social Security Scheme, which covered only 9% of the population. With universal coverage fully implemented, almost 90% of the population is covered (14).
Sex education has been the largest contribution to fighting STI’s in Thailand. In 1991, Thailand created the “100% Condom” program. The Royal Thai government stated that condom use was mandatory for female sex-workers and their customers. The program also consisted of intensive advertising, condom manufacturing and condom distribution. The program further promoted condom use with the Army, local governments, and commercial sex-workers (15). The combination of public health and social marketing made the program extremely effective. Although the program was a direct response to the growing problem of HIV/AIDS in Thailand, it has been shown there is a direct relationship between condom use and decreased prevalence of gonorrhea and other STI’s. In a study of sex workers in the northern province of Tak, rapid increases in condom use with clients occurred simultaneously with a two-fold reduction in gonorrhea rate (16). Gonorrhea rates and condom use with clients in sex workers in Tak province (16).
Although a government mandate for 100 percent condom use in brothels originated early 1990’s, the prevalence of gonorrhea today indicates less than full compliance (17). In fact, in 2007 a Thailand national survey revealed 50 percent of those surveyed did not wear condoms when engaging in sexual intercourse. Furthermore, the head of the AIDS Cluster Division, Somyot Kittimunkong said the majority of the vocational school girls sampled were infected with gonococcal urethritis, a condition caused by Neisseria gonorrhoeae. The government has slowed or ended the intensive prevention campaigns it launched with so much vigor previously and the Public Health Ministry has been forced to shut down sexually transmitted disease clinics due to budgetary constraints. This is problematic because they were offering valuable disease-prevention information. Sujittra Prongsang, from the Bureau of Vocational Education Standards and Qualifications at the Education Ministry, has said in order for sexual disease prevention, Thai society must accept that condoms will prevent disease and get rid of the notion that teen sex is an embarrassment (13).
Sex-workers in Thailand are also tested routinely for STI’s. Some of the tests used in Thailand to diagnose gonorrhea are the Rapid Plasma Reagin (RPR) Test, the Veneral Disease Research Laboratory (VDRL) test, the Gram stain (more commonly used for men), and culture media for gonorrhea.
Partner notification or contact tracing has also been established in Thailand for more than 20 years (17). With the growing network of STI services throughout the country, tracing of contacts and their information has been easier. Contact tracing is an important strategy in the control of sexually transmitted infections because it encourages those who are unaware that they had been exposed to an infection to be tested and treated.
Looking to the future
Unlike Thailand, the U.S. has a program implemented entitled the “The Gonococcal Isolate Surveillance Project” (GISP). In this program, antimicrobial susceptibilities of strains of N. gonorrhoeae are routinely monitored in order to establish a baseline for the selection of different therapies (18). Every month, N. gonorrhoeae is collected from the first 25 males to be diagnosed with urethral gonorrhea in each of the 28 participating U.S. clinics. These are then plated on agar and the susceptibility is tested against the major antibiotics including penicillin, tetracycline, spectinomycin, ciprofloxacin, ceftriaxone, cefixime, and azithromycin. This way, the CDC can recommend what would be the most effective drug therapy at the moment. If Thailand were to implement such a program as this, it would be much easier to determine the resistant strains that are being passed around by the rampant and relatively unmonitored sex industry.
The most effective possibility for full treatment would be to develop a vaccine against gonorrhea. There are no such vaccines at the current time; however, according to the National Institute of Health (NIH) in the U.S., a vaccine is being researched (19). The entire genome of Neisseria Gonorrhoeae has been sequenced by the NIH and will be extremely useful in production of a vaccine because more research can be done on the function of N. gonorrhoeae. The difficulty with manufacturing a vaccine for gonorrhea lies in the fact N. gonorrhoeae does not contain a capsule (21). Researchers must then focus on the bacterium’s outer surface structures as a means of destroying; however, the bacterium has the ability to quickly change its’ surface structures from strain to strain. However, recent research has made coming to a vaccine closer. As mentioned previously, N. gonorrhoeae uses transferring binding proteins to get human iron sources. There is some research currently being done to target these proteins because they do not change much between strains (21). Although Thailand has the most resistant strains, these strains are beginning to spread to the U.S. This spreading is creating awareness and urgency to produce a vaccine. Producing a vaccine is not feasible in Thailand itself due to the fact that Thailand is considered a third world country and simply does not have the resources like the U.S. to create such a vaccine.
Currently, the U.S. is also researching ways to produce a microbicide that can be applied prior to a sexual encounter in order to kill the bacterium before it infects (19); however, a recent study for the microbicide Tenovir (20) was ineffective. If a microbicide were to go on the market, it would be indispensible for the sex industry in Thailand. Because many sex workers are unable to ask their partners to wear condoms, a microbicide could be an essential tool in preventing gonorrhea from spreading.
Gonorrhea is still alive, evolving, and well in Thailand. Although Thailand had initially taken strides to combat gonorrhea, the lack of funding and awareness has created a situation where gonorrhea is allowed to thrive. Further research in western countries will prove to be the saving grace for developing countries such as Thailand in drastically improving their situation. Unless Thailand adopts a new campaign and attitude towards sexually transmitted diseases, it is likely that new strains of antibiotic resistance will continue to rise.
References
1. "Neisseria_gonorrhoeae." European Bioinformatics Institute. Web. <http://www.ebi.ac.uk/2can/genomes/bacteria/Neisseria_gonorrhoeae.html>.
2. Todar, Kenneth. "Pathogenic Neisseriae: gonorrhea and meningitis." Online Textbook of Bacteriology. Web. <http://textbookofbacteriology.net/neisseria.html>.
3. Kaku, Michio. How Science will revolutionalize the 21st century and beyond. 1999. Print.
4. Slonczewski, Joan L., and John W. Foster. Microbiology An Evolving Science. New York: W. W. Norton, 2008. Print.
5. Wong, Brian, et al. "Gonococcal Infections." EMedicine. 21 Apr. 2009. Web.
6. "Gonorrhea - STD information from CDC." Centers for Disease Control and Prevention. Web. 28 Aug. 2009. <http://www.cdc.gov/std/Gonorrhea/>.
7. Falsetta, Megan L., et al. "Transcriptional Profiling Identifies the Metabolic Phenotype of Gonococcal Biofilms." Infection and Immunity 77.9 (2009): 3522-532. Print.
8. Duncan, JA, et al. “Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome” The Journal of Immunology, 2009, 182(10): 6460 -9. Print.
9. "STD Facts - Gonorrhea." Center for Disease Control and Prevention. Web. 27 Aug. 2009. <http://www.cdc.gov/std/Gonorrhea/STDFact-gonorrhea.htm>.
10. Clendennen, T.E., et al. “Antibiotic Susceptibility Survey of Neisseria gonorrhoeae in Thailand.” Antimicrobial Agents and Chemotherapy. 36 (1992): 1682-1687. Print.
11. Crum, John W., et al. “Resistance to Penicillin and Identification of Penicillinase-Producing Neisseria gonorrhoeae Among Clinical Isolates in Thailand.” Antimicrobial Agents and Chemotherapy. 18 (1980):360-361. Print.
12. Trees DL, et al. “Multiclonal increase in ciprofloxacin-resistant Neisseria gonorrhoeae, Thailand, 1998-1999.” Sexually transmitted diseases. 11 (2002):668-73. Print.
13. U.S. Center for Disease Control and Prevention. “Thailand: STD’s are Spreading Fast Among School Girls.” CDC HIV/Hepatitis/STD/TB Prevention News Update. 2007. Print.
14. Rajitkanok A. Puenpatom. Robert Rosenman. “Efficiency of Thai Provincial Public Hospitals during the Introduction of Universal Health Coverage Using Capitation.” Health Care Manage Sci. 11(2008): 319-338. Print.
15. Kenrad E. Nelson. Carolyn Masters Williams. Heil M.H. Graham. “Infectious Disease Epidemiology: Theory and Practice.” Jones and Bartlett Publishers, Inc. 2006: 641. Print.
16. Swaddiwudhipong W, Chaovakiratipong C, Siri S, Lerdlukanavonge P. Sociodemographic “Characteristics and Incidence of Gonorrhoea in Prostitutes Working near the Thai-Burmese border.” The Southeast Asian journal of tropical medicine and public health. 1990 Mar;21(1):45-52. Print.
17. Limpaphayom, Kobchitt. "Status of STD Screening, Diagnosis and Treatment in Thailand." Issues in Management of STDs in Family Planning Settings. 62-62. Print.
18. “Gonoccocal Isolate Surveillance Project” Center for Disease Control and Prevention. Web. <http://www.cdc.gov/std/gisp/>.
19. "Gonorrhea Research." National Institute of Allergy and Infectious Diseases. Web. 28 Aug. 2009. <http://www3.niaid.nih.gov/topics/gonorrhea/research/research.htm>.
20. Wood, BJ, et al. “The Microbicide Tenofovir Does Not Inhibit Nucleic Acid Amplification Tests for Detection of Chlamydia trachomatis and Neisseria gonorrhoeae in Urine Samples.” J Clin Microbiol. 2008 February; 46(2): 763–765. Print.
21. Podosyan, Gevorg A. "Progress made toward gonorrhea vaccine." Web. <http://www.health.am/ab/more/progress_made_toward_gonorrhea_vaccine/>.
Edited by Celin Cuadra , Erica Everett , Midori Higashi , Michael Hsu , and Tiffany Tseng, students of Rachel Larsen