H5N1 Infection and Transmission
By Sofia Elizarraras
Introduction
Avian influenza A, or avian flu, is an Influenza A virus that currently poses a large threat to global health and safety.[1] It is often referred to as a highly pathogenic avian influenza, or HPAI, as it is highly contagious and can be deadly, especially for domesticated poultry. There are several variants of avian influenza, but there is a specific strain that is presently gaining the most attention in the media, referred to as H5N1. The H5N1 variant is known for its severe threat to public health. Additionally, H5N1 has already affected the poultry industry and economy drastically, through increased cost of eggs in grocery stores from decreased egg production (Discussed further in Section 5). [2] Avian flu is known to spread very quickly through domestic poultry flocks and wild aquatic birds, and H5N1 is no exception. One of the greatest risks posed by avian influenza is that although it typically seeks a host in birds, it does possess the ability to be transmitted to other animals. H5N1 specifically is being treated with urgency by public health officials due to its rapid spread, increased levels of mammal pathogenicity, and potential for person-to-person transmission. [3], [4] Although human-to-human transmission is rare, it is possible and growing in number of cases, which makes H5N1 even more important to study before it is too late. Research on H5N1 helps us to better understand influenza as a whole, as well as prevent any future pandemics not unlike that of the 2020 Coronavirus outbreak. As such, this page will have a specialized focus on transmission and infection of H5N1 to mammals, to tie it into current research and concerns of the public health community.
Biological Properties
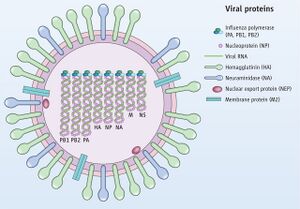
Influenza A viruses belong to the genus
Alphainfluenzavirus and the family
Orthomyxoviridae. [2] They are negative-sense RNA viruses with an eight-segmented, single-stranded genome. The eight-segmented genome is comprised of ten different proteins: neuraminidase (NA), hemagglutinin (HA), nonstructural (NS) proteins NS1 and NS2, the nucleocapsid and the three polymerases, the PB1 (polymerase basic 1), PB2, matrix proteins M2 and M1, and polymerase acidic proteins (See Figure 1). This segmented genome allows for genetic reassortment when two influenza viruses infect the same cell, allowing the genome to avoid host immune response by way of an antigenic shift. Genetic reassortment in influenza A is what makes it possible for a variety of subtype combinations to occur, as discussed below. Influenza type A viruses can be further categorized based on their HA (hemagglutinin) and NA (neuraminidase) antigens, which are the two proteins on the surface of the virus. In total, there are 18 different hemagglutinin subtypes (H1 through H18) and 11 different neuraminidase subtypes (N1 through N11), but only 16 and 9 of these respectively are found in aquatic birds.[5] Out of these subtypes, only a select few have successfully transmitted into mammals. For example, the subtypes documented in humans include H1, H2, H3, N1, and N2, and for pigs, H1, H3, N1, and N2. Due to pigs easily serving as an established receptor for influenza A, they have been considered an intermediate host.
Transmission
Among birds, Influenza A is transmitted in a variety of ways: Birds that are infected with the virus can spread it to others through their saliva, nasal secretions, and feces.[6] It can also be transmitted through direct contact, or indirect contact with surfaces that are contaminated with the virus. For transmission to other animals (including humans), the virus can be transferred through direct contact, or through an intermediate host.[7]

A large factor in determining whether Influenza A will be successful in a host is dependent on the previously mentioned surface protein, hemagglutinin (HA). For humans, virus HAs preferentially bind oligosaccharides that end with a sialic acid and galactose by α2,6-linkages. [8] This is typically seen in the human upper respiratory tract (See Figure 3). For avian influenza, however, the virus prefers to bind oligosaccharides that terminate with sialic acid linked to galactose by α2,3-linkages– this is expressed in the gastrointestinal tract of birds, especially waterfowl. Based on this, it is thought that a shift of HA from α2,3 Sia- to α2,6 Sia-specificity would be necessary to properly facilitate avian influenza transmission in humans.
One other determinant of the host range of Influenza A is the PB2 polymerase protein, as shifts in this protein increase virus replication in the mammal respiratory tract at 91° F. This increase in PB2 leads to increased transmission of not only Influenza A, but multiple viruses.
To avoid acquiring the avian flu, the CDC recommends avoiding travel to countries with H5N1 outbreak first and foremost. If within an affected country, it is recommended to avoid poultry farms or anyplace that live poultry are kept. Additionally, they advise avoiding consumption undercooked poultry, and keeping hands clean after touching raw poultry.
Diagnosis
For domestic birds, H5N1 can be detected by an initial PCR test, and then confirmed by a virus isolation confirmatory test.[9] Samples are obtained by swabbing mucus from the bird’s throat. With wild birds, a fecal sample can be taken in place of mucus. In humans, Influenza A is detected through laboratory tests in conjecture with exposure history to ill or dead birds. Diagnosis is confirmed through HA-specific PCR assay or by viral culture of a nasopharyngeal aspirate. Travel history is also very important in Influenza A diagnosis, and patients with respiratory illness as well as a history of travel to a country with H5N1 within the last ten days should be tested as soon as possible. For low risk patients, testing is recommended within ten days of symptoms.
H5N1 can express symptoms differently depending on the hosts it inhabits. In humans, the virus can display either no symptoms or mild illness ranging from cough, fever, body aches, sore throat, and runny or stuffy nose.[10] [11] Less common symptoms include seizures, nausea, vomiting and diarrhea. In birds, signs might include lack of appetite, reduced energy or coordination, swelling, diarrhea, respiratory signs (coughing and sneezing), decreased egg production, and death. [12]
Treatment
There has been a variety of prior research on potential treatments, ranging from convalescent human blood products to steroids. For instance, a 2006 influenza pneumonia study in Spain revealed success in transfusing influenza-convalescent human blood products. [13] They report a clinically important reduction in the risk for death and improvements in symptoms, and shared hope that similar studies could be conducted for H5N1. However, this Spain study had limitations in that there were only a few studies and none randomized, so future studies should expand and improve this methodology to be better equipped for avian influenza research.
There has been limited success in other trials, and for now there is currently no widely accepted treatment for animals infected with the H5N1 variant. Mild cases can recover in a few weeks with disappearance of symptoms, but in the more extreme cases, such as in turkeys and chickens, the whole flock may be compromised. For the most part, treatment consists of isolating the infected individual and striving to keep the external environment clean for the rest of the flock.
For human cases, however, the CDC recommends treatment with neuraminidase inhibitors for infection. Oseltamivi, also marketed under the tradename Tamiflu, is one such neurominidase inhibitor that can be prescribed for H5N1. The dosage is dependent on the strain's replication rate. They also recommend beginning treatment with flu antivirals as soon as possible after arrival of symptoms. A local health provider should also be contacted immediately to test for avian flu and assist with prescriptions. [14] The United States federal government does have a stockpile of vaccines that can be used against H5N1 (as well as H7N9), but they are reserved for use in the event that the virus begins rapidly transmitting between people. One of the concerns regarding H5N1 vaccine development is regarding the cross-reactivity of H5N1 and memory T cells in healthy individuals, as this combination can be a determinant in how severe the virus is exhibited in the host. [15]
History of Mammal Infection & Transmission
Avian flu within birds has been a focus of research for years now, but a less researched area is the virus’ capability to transmit and infect mammals. Reportedly, most mammalian outbreaks coincide with avian outbreaks. This is of concern because viruses with the ability to jump species barriers can be responsible for influenza pandemics. Examples of mammals that have previously been infected by H5N1 on a global scale include tigers, leopards, Asiatic golden cats, domestic cats and dogs, pigs, martens, red foxes, civets, and donkeys. [16]
From a historical perspective, the initial 1997 Hong Kong H5N1 outbreak in chickens coexisted with the first reported human case of avian flu. [17] This outbreak resulted in the infection of eighteen individuals, including six deaths. As of January 5, 2023, there have been 868 confirmed human cases and 457 deaths from H5N1 infection. Although 868 cases can be considered a small amount in the grand scheme of viruses, the estimated case fatality rate is 53%, making the H5N1 virus one of the most lethal viruses to date.
Current Research
Humans:
Occurrences of humans aquiring H5N1 are quite current and widespread across the globe. For instance, there was a positive case in China as recent as March of 2023. [18] [19] During the same month, a middle-aged man tested positive in Chile. [20] Earlier this year, Ecuador confirmed a case of human transmission in a nine-year-old girl. [21] In February 2023, a father and daughter duo tested positive for the variant in Cambodia, and the daughter died. [22] Although several of the cases reported close contacts, those specific individuals tested negative for the variant.

Non-human Mammals:
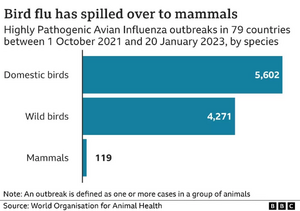
Within the last two years in the United States specifically, there have been 154 reported instances of the H5N1 strain in mammals, commonly foxes, possums, skunks, and harbor seals (See Figure 5). In March, Chile reported more than 500 sea lions died due to H5N1, which followed an outbreak in Peru that killed 3,500 sea lions in addition to thousands of seabirds. [23] Last fall, there was a large outbreak of H5N1 at a Spanish mink farm that killed more than 50,000 mink. [24] None of the human workers were affected, but the rapid rate of transmission amongst the small mammals was very concerning for scientists.
One of the more rarely affected species is the American Black Bear, which aquired H5N1 only recently. For the first time in the United States, a black bear tested positive for the highly pathogenic avian influenza in October 2022. [25] . Although this bear was found in Alaska, there were two more confirmed cases in North Carolina and Colorado in the two months following. This illustrates the severity of H5N1 transposing to new hosts in real time.
Social and Economic Implications

Should another pandemic occur, economic and social costs will inevitably follow disease and death. Huremović D. (2019, p 7) states “In a long succession throughout history, pandemic outbreaks have decimated societies, determined outcomes of wars, wiped out entire populations but also paradoxically cleared the way for innovation and advances in sciences (including medicine and public health), economy, and political systems.” [26] Even without a decreed pandemic outbreak, poultry farmers already feel the strains of avian flu, due to the substantial losses in their flock and the prices of keeping their current birds healthy. Secondarily, the prices of eggs and chicken are skyrocketing in the grocery store due to decreased egg production, which takes tolls on shoppers all over the world (See Figure 6). [27] By the end of 2022, more than 43 million hens were killed by avian influenza since the outbreak started in February of that same year. As of 2008, the World Bank estimates that a human-to-human outbreak of H5N1 will result in economic losses equating to $2 trillion USD. [28] Furthermore, should a global pandemic occur with human-to-human transmission, we will most likely be facing a new set of challenges similar to the panics felt with the SARS-CoV-2 outbreak– not only in economic terms, but also in societal terms through our efforts to slow the spread.
Conclusion
As outlined by this page, there is still a lot of work to do to fully understand influenza and H5N1 in particular. In fact, we may never perfectly understand this virus. H5N1 serves as a real-life example of a public health mystery, by demonstrating how little is known about factors of human influenza pathogenicity and transmissibility, arguably two of the most important questions in infectious disease research. The HPAI H5N1 variant will inevitably continue to gain traction in the media and in the eyes of researchers around the world due to its potential to infect humans, and for its pandemic potential should it acquire the ability to transmit between humans, and it will be interesting to watch this phenomenon unfold over our generation. As for now, we can only hope to keep up with it scientifically as it continues to unravel our wildlife, livestock, and potentially humans.
References
- ↑ “H5N1 Bird Flu: Current Situation Summary.” Centers for Disease Control and Prevention, 2023.
- ↑ 2.0 2.1 Peiris, J. S., et al. “Avian Influenza Virus (H5N1): A Threat to Human Health.” Clinical Microbiology Reviews, vol. 20, no. 2, 2007, pp. 243–267.
- ↑ "Probable Person-to-Person Transmission of Avian Influenza A (H5N1" New England Journal of Medicine.
- ↑ “Past Examples of Probable Limited, Non-Sustained, Person-to-Person Spread of Avian Influenza A Viruses.” Centers for Disease Control and Prevention, 27 Feb. 2023.
- ↑ “Type of Influenza Viruses" Centers for Disease Control and Prevention, 29 March, 2022.
- ↑ “Bird Flu in Birds"Centers for Disease Control and Prevention, 09 March, 2022.
- ↑ “Spread of Bird Flu Viruses Between Animals and People" Control and Prevention, 24 Ma7, 2022.
- ↑ Imai, M., et al. “Transmission of Influenza A/H5N1 Viruses in Mammals.” Virus Research, vol. 178, no. 1, 2013, pp. 15–20.,.
- ↑ Influenza (Avian) Fact Sheet - Pennsylvania Department of Health.
- ↑ Skeik, Nedaa, and Fadi I. Jabr. “Influenza Viruses and the Evolution of Avian Influenza Virus H5N1.” International Journal of Infectious Diseases, vol. 12, no. 3, 2008, pp. 233–238.
- ↑ "Bird Flu in People" Centers for Disease Control and Prevention. 04 May, 2022.
- ↑ Avian Flu Animal Handlers - Occupational Safety and Health Administration.
- ↑ [https://www.acpjournals.org/doi/full/10.7326/0003-4819-145-8-200610170-00139?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org Luke, Thomas C., et al. “Meta-Analysis: Convalescent Blood Products for Spanish Influenza Pneumonia: A Future H5N1 Treatment?” Annals of Internal Medicine, vol. 145, no. 8, 2006, p. 599.]
- ↑ "Treatment of Avian Influenza A Viruses in People" Centers for Disease Control and Prevention. 15 November, 2018.
- ↑ Lee, Laurel Yong-Hwa, et al. “Memory T Cells Established by Seasonal Human Influenza A Infection Cross-React with Avian Influenza A (H5N1) in Healthy Individuals.” Journal of Clinical Investigation, 2008
- ↑ Amonsin, Alongkorn, et al. “Genetic Characterization of H5N1 Influenza A Viruses Isolated from Zoo Tigers in Thailand.” Virology, vol. 344, no. 2, 2006, pp. 480–491.
- ↑ Bryan S., and Richard J. Webby. “The Avian and Mammalian Host Range of Highly Pathogenic Avian H5N1 Influenza.” Virus Research, vol. 178, no. 1, 2013, pp. 3–11 .
- ↑ “Cumulative Number of Confirmed Human Cases for Avian Influenza A(H5N1) Reported to WHO, 2003-2023, 5 January 2023.” World Health Organization, World Health Organization.
- ↑ Avian Influenza A(H3N8) - China.
- ↑ Human infection caused by Avian Influenza A (H5) - Chile.
- ↑ Human infection caused by avian influenza A(H5) - Ecuador.
- ↑ , Apoorva Mandavilli and Emily. “Cambodia Investigates after Father and Daughter Infected with Bird Flu.” The New York Times, The New York Times, 24 Feb. 2023.
- ↑ “2022-2023 Detections of Highly Pathogenic Avian Influenza in Mammals.” USDA APHIS. 17 April, 2023.
- ↑ Wang, Philip. “Explosion of Sea Lion Deaths in Peru amid Deadly Bird Flu Outbreak.” CNN, Cable News Network, 7 Mar. 2023.
- ↑ “Report of Black Bear Cub with Avian Influenza in Glacier Bay National Park (U.S. National Park Service).” National Parks Service, U.S. Department of the Interior
- ↑ Huremović, Damir. “Brief History of Pandemics (Pandemics throughout History).” Psychiatry of Pandemics, 2019, pp. 7–35 .
- ↑ “Avian Influenza Outbreaks Reduced Egg Production, Driving Prices to Record Highs in 2022.” USDA ERS - Chart Detail.
- ↑ “Risk of Bird Flu Pandemic Great despite Efforts.” NBCNews.com, NBCUniversal News Group, 21 Oct. 2008
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2023, Kenyon College
