HIV-1 Pre-Integration Complex
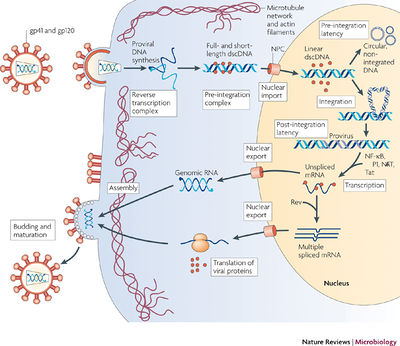
Introduction
Arguably the most prominent and deadly virus discovered in the twentieth century is the human immunodeficiency virus (HIV) due its causative role in the development of the acquired immunodeficiency syndrome (AIDS). HIV is a lentivirus within the Retroviridae family of RNA viruses. Retroviruses use are able to integrate their genomes into that of the host by using the enzyme reverse transcriptase to synthesize a double stranded DNA from their RNA. Furthermore, lentiviral infections are known to progress slowly due to the virus’ long latent period [1]. And one unique aspect about lentiviruses is their ability to infect non-dividing cells [2]. The HIV life cycle consists of viral entry, integration of viral genome into host DNA, transcription and translation of viral genome, virion assembly, and the budding of virions. The successful entry into a target cell involves three main steps: HIV envelope proteins binding to CD4 receptors; binding to co-receptors, CCR5 and CXCR4; and virion fusion or endocytosis [3]. After virion fusion, the core of the virus gets released into the cytoplasm. Once inside the cytoplasm, reverse transcriptase synthesizes double stranded DNA from the RNA genome within the intact core [1]. Moreover, a key feature of the HIV-1 virus is its ability to integrate its genome into the genome of host cells. This process can lead to a latency and mitosis-independent replication of the virus when the viral genome is integrated into heterochromatin and transcription is repressed by histone deacetylation [3]. During the clinical latency stage, HIV viral loads are extremely low and most people do not experience any HIV-related symptoms.
For integration to occur in the nucleus, the virus must successfully cross the nuclear membrane. HIV-1 establishes the pre-integration complex (PIC) that is responsible for nuclear import. The events from when the viral genome gets reverse transcribed to the establishment of the PIC is still unclear. Nevertheless, the PIC consists of the cDNA, integrase (IN), reverse transcriptase (RT), matrix antigen (MA), and viral protein R (Vpr). MA and Vpr both contain nuclear localization signals (NLS) that are believed to be important for nuclear import. MA is recognized by importin-α and importin-β. Vpr aids in nuclear import in a different pathway that increases viral infection. Moreover, a region of the cDNA has been shown to be necessary for PIC entry through the nuclear pore [3]. Insight into the elusive mechanisms of PIC formation might provide a novel drug target for the treatment of HIV. In contrast to the views held for HIV infection in the twentieth century, having HIV is no longer a death sentence since drugs can prolong the progression to AIDS. The main treatment for HIV is antiretroviral therapy (ART), which can extend clinical latency stage for up to several decades [1]. Additionally, HIV-infected individuals who take ART and maintain a low viral load can have a normal life and may never progress to AIDS. Therefore, new and innovative drugs are always in demand for the effective treatment of HIV due to its extremely high rate of mutagenesis and fast replication [4].
Nuclear Import: Matrix Antigens and Viral Protein R
In vertebrates, the nuclear pore has a maximum diameter of 120 nm. The nuclear pore can facilitate the movement of molecules smaller than 9 nm to a maximum diameter of 39 nm. This aspect of the nuclear pore is one of the three main challenges a virus must overcome in order to get its genome into the nucleus. The second problem of nuclear import involves the uncoating of the viral capsid or core. This step is crucial since premature capsid uncoating could be damaging to the viral genome. Furthermore, nucleic acids are required to have high densities and be very compact while in the nucleus. The last main issue with nuclear import has to do with the hydrophobic interactions between nucleoporins and the negatively charged nucleic acid. The HIV virus is able to overcome these challenges and infect non-differentiating cells [2].
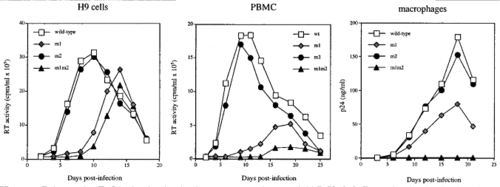
The roles of matrix proteins (MA) and viral protein R (Vpr) in nuclear import is a controversial topic since the roles that these proteins play are still unclear. MA forms a ‘structural shell’ on the inner membrane and is associated with virion assembly and exit. These proteins are formed by the cleavage of the Gag polyprotein by viral protease [5]. One nuclear localization signal (NLS) has been found in the N-terminal region of the Gag protein that encodes MA. Lysine 26 and 27 mutations in this domain was shown to inhibit HIV-1 replication only in non-dividing macrophages but not proliferating cells. Additionally, a C-terminal tyrosine phosphorylation in MA allowed the protein to be integrated into the PIC and might have an effect on viral infection. Although some studies could not find evidence for the existence of NLS in MA, others have reported its presence [2].
Two NLSs have been found responsible for nuclear import regulation in HIV-1 MA. These two NLSs occur in a basic region of MA [6]. The first NLS (NLS-1) occurs from residues 24 to 31, 24GKKKYKLKH [2][6]. This signal acted similar to a nuclear import signal and an HIV-1 strain that had a mutation in this NLS was not able to infect non-differentiating cells. These results were associated with the mutant’s inability to form 2-LTR circles. The other NLS occurs in residue 110 of the C-terminal, 110KSKKK (NLS-2). Mutations within both of these NLSs were shown to inhibit viral replication in macrophages. However, these mutations did not result in the abolishment of nuclear import. Thus, the importance of MA in nuclear import is still unclear [7]. Furthermore, a nuclear export signal (NES) has been found that is involved in viral infection. This NES was shown to have a larger impact on the virus I terms of being able to override the NLS signal [2]. Haffar et al. presented that NLS-1 was the dominant NSL in directing nuclear import. Mutations by replacing the lysine with alanine residues resulted in the absence of nuclear import in both NLSs. Furthermore, mutation in NLS-2 did not abolish nuclear import but mutations in both NLS-1 and NLS-2 resulted in the failure of the mutants to enter the nucleus. Additionally, NLS-1 and NLS-2 mutants did were not able to replicate in macrophages, indicating that MA is important of macrophage infection. NLS-2 was shown to have a binding affinity for karyopherin-α [6]. Karyopherins α are a group of proteins that bind with NLA and are directly involved in nuclear import. Karyopherin-β targets karyophile-karyopherin α complex and aids in this interaction by increasing the binding affinity of karyopherin-α for NLS. Additionally, karyopherin-β is involved in the docking of the viral genome to the nucleoporin [8]. Thus, interactions between MA NSL-2 and karyopherin-α suggest that MA plays an important role in nuclear import of HIV-1 genome.
Aside from MA, Vpr is a transcription factor and is responsible for DNA import across the nuclear membrane. Vpr is also involved in apoptosis and G2 cell cycle arrest (slonc). One study observed that HIV-1 virus with NLS-1 MA mutations was able to replicate in the presence of Vpr and viral replication was only inhibited when there were mutations in both MA and Vpr [rijck]. Here, these results imply the major role that Vpr could play in nuclear import. The secondary structure of Vpr consists of three α helices at residues 17-33 of the N-terminal region, at residues 35-50, and resides 55-77 of the central region [rijck, haffar]. The first α helix structure on Vpr has been shown to mediate the docking of the PIC to the nuclear pore complex (NPC) via specific regions on nucleoporins, such as phenylalanine-glycine reapeat sequences. In addition, the protein exhibits some interactions with importin α, which also interacts with MA and integrase. There are two different ways that Vpr interacts with importin α. One possibility is that importin α binds to PIC via MA or integrase while Vpr binds to importin α to dock the PIC at the NPC. In the other pathway, Vpr aids in nuclear import by stabilizing importin α and PIC binding [7]. Vpr seems to play a very important and complex role in nuclear import. Vpr might contain two non-classical NLS, one located in the N-terminus and the other in the C-terminus. When a fusion protein was used to localize the protein to the nucleus, first α helix and the third α helix of the protein showed translocation to the nuclear pore [7].
Moreover, Mutations of the α helices can affect Vpr incorporation into virons. Mutation of the N-terminal α helix affects the cell cycle progression and mutation of the center α helix affects the nuclear translocation. Vpr also contains an arginine-rich C-terminal. Mutations within this region affects nuclear localization and some cell cycle components. There are three hypotheses about regarding the role of Vpr in HIV-1 nuclear import. One model proposes that Vpr regulates PIC nuclear import via a karyopherin-α independent pathway since Vpr-facilitated nuclear import was resistant to a non-functional karyopherin-α. The second hypothesis suggests that Vpr requires karyopherin α but not β to successfully dock the PIC at the nuclear pore. Lastly, the Vpr might bind to karyopherin α to improve the karyopherin-α and NLS interaction and overall enhances karyopherin-dependent nuclear import [6].
Although the functions of Vpr remain unclear, the protein has been shown to greatly increase the infectivity of HIV-1 in non-dividing macrophages. One study reported that while mutations in the MA NLS only decreased nuclear import, the absence of Vpr in mutant viruses exhibited the complete absence of nuclear import activity. The researchers also found while MA has to be a component of the PIC to perform its functions, Vpr can effectively regulate nuclear import without being a part of the PIC. Similar to MA, Vpr was seen interacting with karyopherin-α. In contrast, Vpr did not bind to karyopherin-α via NLS like MA. Since karyopherin-α binding by Vpr is NLS-independent, Vpr can bind to karyopherin-α at the same time as MA and decrease competitive inhibition by NLS and act as a regulator to increase the binding affinity of MA to karyopherin-α. Furthermore, Vpr can increase the nuclear import of artificial weak karyophiles but at high concentrations can inhibit the nuclear import of all artificial karyophiles. This study presents a model for the nuclear import of PIC where Vpr binds to karyopherin-α and increases its binding affinity to MA via NLS. The increase in affinity increases the ability of PIC to compete for karyopherin-α/β heterodimers and facilitate the translocation of the PIC through the nucleus pore. In spite of these findings, although Vpr enhances nuclear import and is important for viral infection, it is not necessary for HIV-1 infection [9].
HIV-1 cDNA and the reverse transcription complex
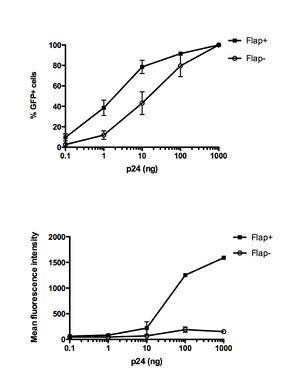
The cDNA in the PIC contains a DNA flap or the triple helical domain. The HIV-1 genome contains a central polypurine tract (cPPT) and central termination sequence (CTS) that results in the DNA flap during the synthesis of the positive-strand DNA by reverse transcriptase. Initiation of reverse transcription occurs at the cPPT. All lentiviruses contain two cis-acting sequences, a central polypurine tract (PPT) in the coding sequence for integrase and a PPT in the 3’region that is resistant to RNase H degradation. The 3’PPT functions as a primer for the positive strand synthesis. Initiation of reverse transcription occurs at both the cPPT and the 3’PPT, which leads a break in the center of the viral genome. Furthermore, reverse transcription terminates 100 nucleotides downstream of the cPPT in the CTS. At this region, the conformation of the CTS reduces the binding ability of reverse transcriptase and leads to termination. From these events, the DNA flap is generated from the 100 nucleotide overlap. Mutations in the cPPT and the CTS results in the impairment of HIV replication and the DNA flap. One study introduced extensive mutations in the cPPT and found that infectivity decreased in the mutants. And when the cPPT mutations were combined with CTS mutations, infectivity further decreased. However, disruptions in the cDNA flap formation does not completely abolish nuclear import since about 5-15% of these mutants exhibited nuclear import and infectivity. From these results, it is unclear whether the observed infectivity of the mutants is due to an incomplete DNA flap disruption or there exists a mechanism for DNA-flap independent nuclear import[10]. One possibility for this mechanism is that smaller viral genomes are able to pass through the nuclear membrane without a central DNA flap[10][11].
For the PIC to cross the nuclear membrane, it must adopt an extreme conformation. One hypothesis have been proposed for the mechanism of DNA flap- dependent nuclear import. In this hypothesis, after the successful reverse transcription of the viral genome within the viral capsid and the docking of the reverse transcription complex (RTC) to the nuclear pore, RTC undergoes maturation into the PIC. Afterwards, the central DNA flap would translocate outside of the integral viral capsids. Without the large HIV capsid, the viral genome can enter the nuclear pore. In this model, mutants with the DNA flap would prevent the cDNA from translocating outside the viral capsid. Another model hypothesizes that the DNA flap constitutes a part of the PIC and integrase and is necessary for the recognition of the PIC by the nuclear pore. In the presence of cPPT mutants, the nuclear pore machinery would not recognize the PIC and nuclear import would not occur [11].
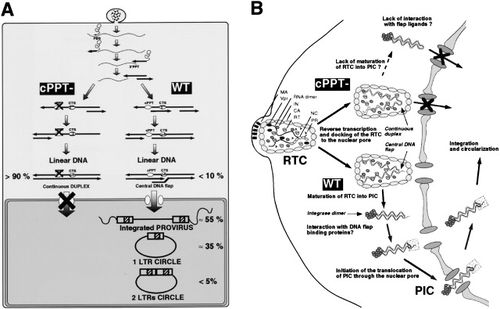
There is evidence that the viral capsid remains intact until the entire complex moves to the nuclear membrane. This means that reverse transcription occurs while the viral genome is within the viral capsid[10][12]. In contrast, it has also been reported that the capsid proteins are associated with the viral genome in the cytoplasm, indicating that reverse transcription occurs in the cytoplasm. However, it is possible that the latter observation is due the damage to the capsid from the isolation protocol. Furthermore, the argument for reverse transcription occurring inside the viral capsid is more compelling since the reverse transcriptase enzyme cannot carry out its functions in the high dilution environment of the cytoplasm [12].
Integrase and LEDGF/p75
Integrase is responsible for the integration of the viral genome into the host DNA. Although integrase is a key enzyme in the events following PIC crossing into the nucleus, it also might play a role in the PIC nuclear import. After PIC enters the nucleus, the viral DNA can undergo two different pathways. In one pathway, the viral genome circularizes by integrating onto itself and this does not produce any infective virions. The second pathway is to integrate its genome into the host, which leads to latent infection. The integrase protein contains three domains: N-terminal, core, and C-terminal. The N-terminal domain is within residues 1 to 50 and contains a zinc-binding motif. This domain has been shown to increase the activity of the enzyme. Another important region is the core domain, residues 50 to 212, and is marked by the presence of a highly conserved DD35E motif. This region is involved in catalysis by binding to viral and host DNA. The last domain from residues 218 to 288 is the C-terminal domain that is important for multimerization [7].
HIV was reported to not contain transferable NLS. Furthermore, the nuclear localization of integrase depended upon Lens Epithelium-Derived Growth Factor (LEDGF/p75). This endogenous molecule is a nuclear transcriptional co-activator. In lentiviruses, LEDGF/p75 directs integrase to the host nucleus and tethers the enzyme to the host chromatin. Additionally, the absence of LEDGF/p75 results in the exclusion of HIV-1 integrase from the nucleus. This information indicates that LEDGF/p75 is the main regulator of lentiviral integrases. [ sande] Furthermore, LEDGF/p75 is thought to be responsible for chromosomal tethering of integrase to the nucleus. This growth factor binds to the core domain of integrase with its C terminus and binds to the chromatin with its N terminus. Overall, the role of LEDGF/p75 in integrase tethering suggests its role in mediating the nuclear import of PIC [7].
The integration of the viral genome occurs in three main reactions: processing reaction, joining reaction, and disintegration reaction. In the processing reaction, integrase cleaves the ends of the viral DNA to reveal a 3’-OH group at the ends of the strands. The 3’-OH end acts as the nucleophile in the attack of the 5’ target site on the host DNA. After this step, the joining process involves a coordination of cleavage and ligation reaction in which the two viral DNA ends attack the DNA backbone. This results in single-stranded gaps in the host DNA, which are filled in by the gap repair machinery. The third reaction is a reverse of the joining reaction, in which a molecule intended for integration is broken down into viral and other components [13].
Drug Implications and Future Research
OHIV disease is marked by CD4 T lymphocyte levels of 500/mm3 and higher. While CD4 levels below 200/mm3 are termed as AIDS. At this advanced stage in HIV infection, patients are at risk of opportunistic infections. Antiretroviral (ARV) treatment targets many stages of the virus’ life cycle, including reverse transcriptase and integrase action. There are two different types of drugs that are used to block RT, nucleosides (NRTIs) and non-nucleosides (NNRTIs). The NRTIs are structural analogs of normal nucleosides/nucleotides while NNRTIs do not have this structure but bind to RT’s active site. Initial ARV treatments consist of two NRTI drug combinations as the “backbone drug” and one “cornerstone drug.” The latter drug can be an NNRTI, a protease inhibitor or an integrase inhibitor. Moreover, there are many dual NRTI combinations such as Tenofovir or Lamivudine with emtricitabine or Abacavir with Lamivudine. In contrast, the only integrase inhibitor currently available is Raltegravir. This drug is very potent and has a few reported side effects [3].
To target the PIC, it would be useful to use drugs not only against RT and integrase but Vpr and MA proteins. HIV responds extremely well to antiretroviral treatment such that one potent drug within the arsenal of AZT drugs can drastically reduce viremia loads and extend the patient’s progression to AIDS.
References
[1] Slonczewski, Joan, and John Foster. "Microbiology: An Evolving Science" February 20, 2008. W.W. Norton and Company, New York. http://www.wwnorton.com/college/biology/microbiology2/
[2] Fassati A, Goff SP: Characterization of intracellular reverse transcription complexes of Moloney murine leukemia virus. J Virol 1999, 73:8919-25. http://www.retrovirology.com/content/3/1/74
[3] Greene, W. et al. 2013. Molecular biology of HIV: implications for new therapies. Sande's HIV/AIDS. Ch. 3 & 23. http://www.sciencedirect.com/science/book/9781455706952
[4] Holland, J. et al. 1982. Rapid evolution of RNA genomes. Science. 215, 1577–1585. http://www.ncbi.nlm.nih.gov/pubmed/7041255
[5] Hill, C. P., Worthylake, D., Bancroft, D. P., Christensen, A. M., & Sundquist, W. I. (1996). Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proceedings of the National Academy of Sciences of the United States of America, 93(7), 3099–3104. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC39768/
[6] Haffar OK, Popov S, Dubrovsky L, Agostini I, Tang H, Pushkarsky T, Nadler SG, Bukrinsky M: Two nuclear localization signals in the HIV-1 matrix protein regulate nuclear import of the HIV-1 pre-integration complex. J Mol Biol 2000, 299:359-68. http://www.ncbi.nlm.nih.gov/pubmed/10860744
[7] De Rijck J, Debyser Z. 2006. The central DNA flap of the human immunodeficiency virus type 1 is important for viral replication. Biochem Biophys Res Commun 349:1100 – 1110. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC114123/
[8] Nadler, S.G., Tritschler, D., Haffar, O.K., Blake, J., Bruce, A.G., Cleavland, J.S., et al. (1997). Differential expression and sequence-specific interaction of karyopherin alpha with nuclear localization sequences. J. Biol. Chem., 272, 4310–4315. http://www.ncbi.nlm.nih.gov/pubmed/9020149
[9] Popov, S., Rexach, M., Zybarth, G., Reiling, N., Lee, W. A., Ratner, L., et al. (1998). Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. Embo Journal, 17(4), 909-917. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1170440/
[10] Iglesias, C., Ringeard, M., Di Nunzio, F., Fernandez, J., Gaudin, R., Souque, P., et al. (2011). Residual HIV-1 DNA flap-independent nuclear import of cPPT/CTS double mutant viruses does not support spreading infection. Retrovirology, 8, 92. http://www.retrovirology.com/content/8/1/92
[11] Arhel, N. J., Souquere-Besse, S., Munier, S., Souque, P., Guadagnini, S., Rutherford, S., et al. (2007). HIV-1 DNA flap formation promotes uncoating of the pre-integration complex at the nuclear pore. Embo Journal, 26(12), 3025-3037. http://www.ncbi.nlm.nih.gov/pubmed/17557080
[12] Zennou, V., Petit, C., Guetard, D., Nerhbass, U., Montagnier, L., & Charneau, P. (2000). HIV-1 genome nuclear import is mediated by a central DNA flap. Cell, 101(2), 173-185. http://www.ncbi.nlm.nih.gov/pubmed/10786833
[13] Llano, M., Vanegas, M., Fregoso, O., Saenz, D., Chung, S., Peretz, M., et al. (2004). LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral preintegration complexes. Journal of Virology, 78(17), 9524-9537. http://www.ncbi.nlm.nih.gov/pubmed/15308744
