How E. aerogenes Affects Circadian Rhythm
Circadian Rhythm
By Olivia Smith
Circadian rhythm is a natural endogenous process that regulates the sleep-wake cycle in an organism [1]. This clock cycle is driven by an internal diurnal oscillator set for a period of 24 hours [2]. Circadian rhythms regulate behavior, organs, specifically the gut, and cells in living organisms [3]. External environmental cues, including light, temperature, and redox cycles, can adjust the cycle [1]. Organisms evolved to develop regulatory clock cycles to adapt to the circadian nature of their environment [4]. To competitively survive, as well as optimize function and health, organisms have accommodated rhythmic environmental challenges [1] [4]
. Animals, bacteria, fungi, and plants all exhibit diurnal oscillations of varying degrees of complexity [2]
. This page explains the bidirectional relationship between daily oscillations of gut microbiota and its host.
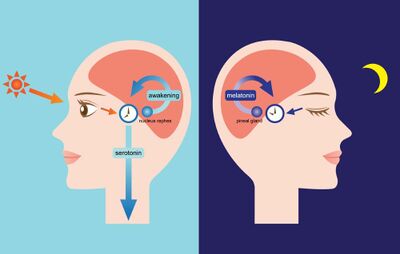
The circadian rhythm is linked to the light/dark cycle of the Earth, which is driven by the suprachiasmatic nucleus of the hypothalamus [5]. In mammals, the suprachiasmatic nucleus is where the primary circadian clock is located [2]. The suprachiasmatic nucleus generates the clock cycle and synchronizes the periphery clock that resides in the tissues of the body [2]. The cycle is synchronized to solar time by retinal afferents from intrinsically photoreceptive retinal ganglion cells [2]. The human eye’s retina contains photoreceptive ganglion cells that are photoreceptive and project to the suprachiasmatic nucleus [2].
Ganglion cells contain melanopsin, which is a photopigment that serves crucial role in the photoentraintment of circadian rhythms [6]. Melanopsin expresses axons that target to the Suprachiasmatic Nucleus [6] [7]. The information from the retinal ganglion cells interprets the lengths of the day and night and passes it to the pineal gland in the epithalamus, this is how mammals interpret the light/dark cycle into their own diurnal oscillations. [7]. The pineal gland secretes the hormone melatonin [2]. Melatonin is an endogenous signal of darkness [5]. Melatonin secretion peaks at night and ebbs during the day [7]. The release of melatonin secretes into the bloodstream and cerebrospinal fluid and conveys signals darkness to receptors all over the body [6].
There are proteins in the suprachiasmatic nucleus that activate the transcription of genes encoding the repressors PERIOD and CRYPTOCHROME [2]. The products of these genes can form a repressive complex [2]. This PER/CRY complex can translocate into the nucleus and inhibit the suprachiasmatic nucleus proteins CLOCK and BMAL1 from transcription activity resulting in PERIOD and CRYPTOCHROME gene repression [2]. This is called the backward limb of the clock. The forward limb of the clock is when BMAL1 and CLOCK are promoted.
Circadian rhythms serve an important purpose. These diurnal oscillations prepare the body for possible changes in the environment, such as activity time, sleep time, and meal time [1]. External cues also serve an important role in maintaining the cycle of the clock [1]. The change in the brightness of the sun is the strongest influence [1]. The future of circadian biology will depend heavily on the application of microbiology techniques to explore the biological time-keeping rhythms. An understanding in circadian rhythms and how the Gut Microbiome interacts with the cycle will be able to answer questions about circadian ailments and respective variations linked to polymorphisms of the clock genes.
Gut microbiome on circadian rhythm
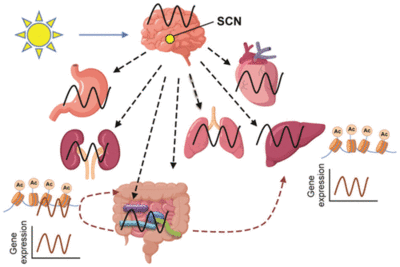
The mammalian gastrointestinal system contains a circadian clock that controls intestinal function [3]. Circadian rhythms regulate intestinal microbiota through both intrinsic clock cycles and the host organism [3]. Microbiota rhythms are regulated by diet and fasting time, which can alter metabolic activity as well as microbiota structure [3]. This can impact the host’s immune and metabolic function [5]. The gut microbiome exhibits circadian oscillation and synchronizes it with the host circadian clock [3].
Bacterial circadian rhythms are the simplest clocks, exemplified by cyanobacteria [8]. Recent research of Synechococcus elongatus, a cyanobacteria, demonstrated that its circadian clock can be reconstituted in vitro with the Kai protein complex of its central oscillator [8]. The first in vitro circadian rhythm was discovered by composing the proteins KaiA, KaiB, KaiC, and the energy source ATP [8]. Previous studies of prokaryotic circadian rhythms were said to be dependent on a DNA transcription/translation feedback loop [7]. Research on fecal microbiota exhibited a difference in the composition of the microbes depending on the time of day [9]. More than 15% of the detected microbes oscillate daily in their relative abundance [9]. These species include Clostridiales, Lactobacillales, Bacteroidales, Firmicutes, and Proteobacteria [9].
Additionally, the total biomass of the gut microbiome is higher during the active phase of the circadian rhythm for the species [9]. During the active phase, 23 % of genes for the pathways involved in energy metabolism, DNA, repair, and cell growth peak in abundance [9]. In contrast, the dark phase shows many of those genes for pathways involved in detoxification, environmental sensing, and motility [9]. These temporal changes help the bacteria anticipate changes in gastrointestinal function. The relationship between daily oscillations of gut microbiota and the host is bidirectional. The host regulates the daily oscillation in the gut microbiome [2]. The daily oscillation of microbial structure requires a functional circadian clock in the host [2]. For example, if the repression of proteins CLOCK and BMAL1 is disrupted, the daily oscillations are abolished in bacterial abundance and metagenomic gene contents [7]. The forward limb of the clock can abolish the circadian rhythmicity of the gut microbiota composition if disrupted [7]. An example of the circadian rhythm disruption via jet lag can shift the gut microbiota [9]. The host circadian clock is fundamental to communicating environmental cues to the gut microbiota [10]. This will help synchronize, coordinate, and adapt between the host and bacteria [9].
Diurnally oscillating microbiota are regulated by both the host and its environment [10]. However, many circadian rhythms rely on environmental cues; they can also be dynamic and persist in the absence of these signals, such as light and temperature [10]. Host-derived melatonin and body temperature are direct regulators of gut microbiome oscillation [11]. When isolated, Enterobacter aerogenes expressed circadian behavior in swarming and motility [3]. This behavior was synced to a 24 hour pattern in the in vitro culture [3]. The oscillations of E. aerogenes were enhanced by melatonin levels [3]. Melatonin is a rhythmically controlled circulating hormone [12]. The isolation of E. aerogenes proved that bacteria within the gut microbiome likely have endogenous circadian clocks that interact with the host circadian clock via signaling molecules, such as hormones [3]. Dietary components and the timing of meals plays a vital role in the circadian oscillation of the gut microbiome [10]. Diet and feeding also influence circadian rhythms of the gut microbiome [10]. High-fat diets cause a lack of daily fluctuation in the relative abundance of Bacteroidetes [3]. Restricting feeding, or fasting, during the light phase rescues the loss of the changes in the gut microbiota [10]. The circadian rhythms of host behavior are entrainable by daily cycles of restricted feeding [10]. During the active phase, the host will be eating, but during the dark phase, the host will be fasting and resting [2]. The gut microbiome also influences the peripheral host circadian rhythm [3]. Peripheral clocks exist in the host’s liver, gut, heart, and retina [3]. Metabolism, a metabolic function in both the host and the microbiome, is intertwined with circadian rhythm[9]. Evidence for oscillation of the gut microbiome and a bidirectional interaction between the gut microbiome rhythm and the host circadian clock has now been well-established.
Enterobacter aerogenes
Enterobacter aerogenes are Gram-negative, indole negative, oxidase negative, catalase-positive, and citrate positive bacterium belonging to the genus Enterobacter [13]. This pathogenic bacterium causes opportunistic infections but is sensitive to most antibiotics. If antibiotic-resistant, it can cause sepsis, a condition in which the body’s response to infection will cause it to injure its own tissues and organs [11]. However, E. aerogenes have inducible resistance mechanisms, such as lactamase, which allows the bacterium to become resistant to standard antibiotics quickly [11]. E. aerogenes has inducible resistance mechanisms, such as its absence of the characteristic inducible Bush group 1 chromosomal beta-lactamase [14]. Lactamase allows the bacterium to become resistant to standard antibiotics such as ampicillin, cephalothin, and cefoxitin [14]. E. aerogenes are generally found in the human gastrointestinal tract but do not cause healthy individuals’ disease [11]. Most Enterobacter infections arise from a patient’s own endogenous gastrointestinal microbiome rather than from the environment [14]. Future research could examine the environmental factors that cause disease to healthy individuals and how to cure the disease if Enterobacter's are antibiotic resistant.
E. aerogenes is in the family Klebsiella, which are found nearly everywhere in nature [15]. E. aerogenes evolved through distinct sub lineages to develop specific niche adaptation [16]. These biochemical adaptations make them better suited to any environment they are found in [15]. They can be found in water, plants, soil, and animals including humans [15]. Dispersion of the E. aerogenes is related to the presence of a redundant regulatory cascade that effectively and efficiently controls membrane permeability to ensure bacterial protection and expression of detoxifying enzymes involved in the degradation and inactivation of antibiotics [13].
Additionally, these E. aerogenes can acquire several genetically mobile elements that contribute significantly to antibiotic resistance [13]. This fitness helps E. aerogenes colonize multiple environments and hosts and quickly and efficiently adapt metabolic and physiological functions to external conditions and environmental stress [13]. E. aerogenes adopts the daily oscillations of its host and responds to the oscillations through external conditions [13].
The energy source of E. aerogenes is hydrogen production and is a facultative anaerobe [13]. This microbe ferments hydrogen through a variety of sugars: glucose, fructose, galactose, etc [13]. E. aerogenes possess a short doubling time and high hydrogen productivity and evolution rate. Its high evolution rate is in part due to efficiently adapting metabolic and physiological functions to stressors [13]. The metabolic behavior and growth of this microbe can also vary under different environmental conditions owing to its diverse metabolites--acids and alcohols-- and its capacity of utilizing a variety of sugars [13]
Enterobacter aerogenes on Circadian Rhythm
Enterobacter aerogenes suggests the existence of indigenous clocks in some microbiota bacterial cells synchronizing with host circadian regulators because of its sensitivity to the host-derived neurohormone melatonin [7]. The bacterial microbiome is an essential modulator for gastrointestinal function [17]. The circadian biological clock plays a crucial role in that process from the perspective of the circadian rhythm of the microbiome by the host clock as well as from the perspective of the microbiota that may influence the gastrointestinal clock [7].
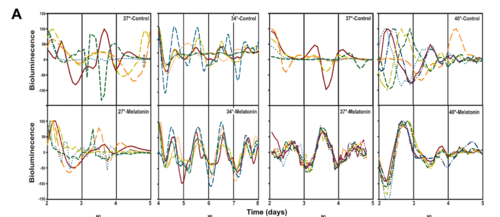
Circadian clocks are fundamental properties of all eukaryotic organisms and some prokaryotic microbes. The mammalian gastrointestinal system contains a circadian clock regulating gastrointestinal function [17]. The gut bacterium E. aerogenes increases swarming activity in response to the pineal and gastrointestinal hormone melatonin [7]. In vertebrates, the gastrointestinal system expresses circadian gene expression patterns, motility, and secretion in vivo and in vitro [7]. The enteric microbiome is regulated by the host’s circadian clock [7]. E. aerogenes are sensitive to the neurohormone melatonin [7]. Melatonin is secreted into the gastrointestinal lumen and expresses circadian rhythms of swarming and motility [12]. Melatonin increases the magnitude of swarming in cultures of E. aerogenes [12]. However, this pattern is not seen in microbes such as Escherichia coli or Klebsiella pneumoniae [7]. The swarming runs on the 24-hour clock of the circadian rhythm [7]. The MotA promoter on flagellar motor-protein driven plasmids transforms E. aerogenes to express luciferase, revealing circadian patterns of bioluminescence synchronized by melatonin and dependent on temperature [11]. Altogether, these data suggest that the human circadian rhythms may regulate the entrainment of bacterial clocks. Disruption of the circadian clock, either via dietary restriction or jet lag, affects the temporal distribution of the gut microbiome community [9]. Cultured colonies revealed that E. aerogenes proliferated more rapidly in the presence of melatonin than without it [7]. Larger cultures of E. aerogenes exhibited swarming patterns and showed patterns of concentric rings that coincided with the number of incubation days [7]. This showed that the swarming rhythms represent the cycle of a circadian clock [7]. Bioluminescence of E. aerogenes showed a significant effect of melatonin on the phase of peak bioluminescence [7]. The presence of circadian rhythms within symbiotic bacteria in response to endocrine signals regulated by the host’s circadian mechanism adds further credibility to the concept of the microbiota as a metaorganism [7]. One has an intrinsic clock swept by the host’s clock drive signal [7]. Considering our own circadian mechanism as an evolved adaptation to environmental phenomena dominated by a 24-hour cycle, organs and organ systems may be perceived as a takeaway environment for resident microbiota [10]. Thus, environmental perturbations, such as circadian perturbations, affect rhythms within the microbial gut, as previously shown [10].
Gut bacteria possess their own daily rhythmicity in their localization and function [17][4]
. Gut bacteria can modulate host rhythms through microbial metabolites such as butyrate, polyphenolic derivatives, amines, and vitamins [17]. As mentioned previously, lifestyle stressors such as altered sleep patterns and eating habits can disturb the host circadian cycle and impact the gut microbiome [17] [4]. Several disruptions in the gut can affect substrate oxidation and energy regulation in the host [17]. Disturbances include decreased conjugation of bile acids or increased production of hydrogen sulfide, resulting in a decrease in butyrate production [17]. Sleep patterns and a healthy diet are essential for maintaining gut microbial balance [17] [4]
. The molecular clock that is found in almost every cell contains the genes Per1, Per2, and Per3, the brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1), cryptochrome (CRYPTOCHROME), and the circadian locomotor output cycles kaput gene (Clock) [17]. This molecular clock regulated the rhythmic expression of clock-controlled genes [5] [17]. These clock-controlled genes regulate the synthesis, storage, and expenditure of energy [17].
Conclusion
The gut microbiome can be influenced by many environmental factors and stressors. As this page has discussed, stressors such as food intake and timing, temperature abnormalities, and sleep patterns can all disrupt the gut microbiome. The gut microbiome has microbes that respond and react to these stressors which then affect other organs in the body, specifically the brain. Circadian rhythms are delicately balanced on many factors as well, one of them being the host gut microbiome. The gut microbiome is subject to the diurnal oscillations and entrainment by host circadian rhythms. In turn, a diverse microbiota is fundamental for the most evolutionarily favorable regulation of host circadian oscillations. A species of human gut bacteria, Enterobacter aerogenes , is a microbe that plays a generous role in the relationship between the gut microbiome and the circadian rhythm. E. aerogenes regulates the diurnal gene transcription by entrainment of the hormone melatonin to the 24 hour cycle of the circadian rhythm. E. aerogenes has its own circadian rhythm and responds to fluctuations of melatonin [11]. The rapid proliferation of E. aerogenes when melatonin was present proves it's holding of a circadian rhythm. The patterns of bioluminescence in cultures of E. aerogenes demonstrated the 24 hour cycle of its oscillations. All the work mentioned indicates that the circadian clock of gut microbiota respond to hormonal signals elicited from the circadian clock of the host.
Although it is now evident that commensal microbial signaling affects maintenance of gut homeostasis and circadian control of intestinal and various extra-intestinal functions, it is still largely unclear through which mechanisms commensal bacteria and host tissues communicate and how the microbiome may take advantage of host circadian functions to maintain its own homeostasis. Despite the exact mechanisms accounting for the observed bacterial rhythms being unknown, future studies can begin with he understanding of the relationship between melatonin and E. aerogenes . Future experiments can research the clock gene and the effects of PERIOD and BMAL1 deletion in the genome [9]. It is also yet to be determined if jet-lag is mediated through the intestinal clock system or the Suprachiasmatic Nucleus with melatonin [9]. Jet lag affects the temporal distribution of the gut microbiome constituents and also affects the light/dark cycle.
Circadian clocks allow gut microbiota to anticipate changes in the environment and to take advantage of predictable factors, such as temperature, light, and food, or to avoid environmental stressors.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "National Institute of Neurological Disorders" 2017. Brain basics: Understanding sleep.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 Heinemann, Ratiner, K., Elinav, E. “Basic Biology of Rhythms and the Microbiome.” 2017. Circadian Rhythms in Bacteria and Microbiomes 317-328.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Liang, FitzGerald, G.A. “Timing the microbes: the circadian rhythm of the gut microbiome.” 2017. J Biol Rhythm 32:505-515.
- ↑ 4.0 4.1 4.2 4.3 4.4 Nobs, Tuganbaev, T., Elinav, E. "Microbiome diurnal rhythmicity and its impact on host physiology and disease risk. 2019. EMBO Rep 20:e47129.
- ↑ 5.0 5.1 5.2 5.3 Voight, Forsyth, C.B., Green, S.J., Engen, P.A., Keshavarzian, A. “Chapter Nine - Circadian Rhythm and the Gut Microbiome.” 2016. International Review of Neurobiology.
- ↑ 6.0 6.1 6.2 Hattar, S., Liao, H.W., Takao, M., Berson, D.M., Yau, K.W. “Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity.” 2002. Science.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 Paulose, Wright, J.M., Patel, A.G., Cassone, V.M. "Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. 2016. PloS one.
- ↑ 8.0 8.1 8.2 Nakajima, M., Imai, K., Ito, H., Nishiwaki, T., Murayama, Y., Iwasaki, H., Oyama, T., Kondo, T. "Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. 2005. Science (New York, N.Y.) 308:414-415.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 Zeevi, D., Levy, M. “Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis.” 2014. Cell.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 Karl, P.J., Hatch, A.M., Aricidiacono, S.M. "Effects of psychological, environmental and physical stressors on the gut microbiota. 2018. Front Microbiol 9:2013.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Cassone, V.M. "The melatonin-sensitive circadian clock of the enteric bacterium Enterobacter aerogenes. 2016. Gut Microbes 7: 424-427.
- ↑ 12.0 12.1 12.2 Reiter, "The photoperiod, circadian regulation and chronodisruption: the requisite interplay between the suprachiasmatic nuclei and the pineal and gut melatonin. 2011. J Physiol Pharmacol.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 13.8 "Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. 2015. Frontiers.
- ↑ 14.0 14.1 14.2 Sanders, W.E., Sanders, C.C. “Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis.” 1997. <Clinical Microbiology Reviews.
- ↑ 15.0 15.1 15.2 Falkow, Stanley "The Prokaryotes Vol. 1: Symbiotic Associations, Biotechnology, Applied Microbiology. 2006. Springer New York 3:159-196.
- ↑ Sanders, Cassone, V.M. "Enterobacter spp.: pathogens poised to flourish at the turn of the century. 1997. Clinical Microbiology Reviews 10: 220-241.
- ↑ 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 Parkar, Kalsbeek, A., Cheeseman, J.F. "Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. 2019. Microorganisms 7:41.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College
