Impact of Exercise on the Gut Microbiome
By Emi Loucks
Intro
Exercise is a common form of health regulation. It is known to bring many positive impacts including weight loss, improved cardiovascular health, improved mental health, symptomatic reduction or prevention of chronic diseases including morbid obesity and inflammatory bowel disease (IBS), and other contributors to overall quality of life. The mechanisms by which exercise stimulates these effects in the body are complex. One increasingly popular area of research over the past decade and a half is the gut microbiome and how the physiological changes may be due to changes in the gut microbial population, in other words, how changes in microbe diversity, species prevalence, and their products affect the rest of the body. Published animal and human studies using both cross-sectional and longitudinal approaches have elucidated certain outstanding correlations [1].
Effects of Exercise on Gut Microbial Health
Routine exercise is correlated with a healthier body. A study by Estaki et al. describes cardiorespiratory fitness as a “better predictor of mortality…than smoking, diabetes, and hypertension” [2]. Specifically, it is thought that a healthier gastrointestinal tract caused by exercise initiates a healthier state of the rest of the body. Beneficial gastrointestinal changes from exercise include a reduction of conditions including diverticular disease (weak protrusions from the colonic wall), cholelithiasis (gallstones), constipation, and colonic cancer [3]. High activity level is associated with a 24% reduced risk of colorectal cancer [1]. Other examples include an observed increase in sympathetic tone in the autonomic nervous system [1]. A study by Meissner et al. compared the bile acid secretion in mice exercised for 12 weeks to a control of sedentary mice and found improved circulation of bile acids in the enterohepatic system [4] [1]. Bile acids are an important regulating factor in the gut microbiome, and a reduction or poor circulation of the acids is associated with gut dysbiosis [1]. Myokines, metabolites, and neuroendocrine hormones are also examples of important molecules for gut health, and exercise benefits their associated metabolic pathways [1]. The pathways of carbohydrate metabolism and biosynthesis of amino acids are improved by exercise as well, which has helped improve IBS symptoms in patients, including Crohn’s disease and ulcerative colitis – both of these conditions are recognized by an unhealthy gut microbiota composition and resulting improper immune responses and a reduction of the aforementioned pathways [1].
Furthermore, typical markers seen in athletes or routinely active persons include a reduction of bacterial endotoxin lipopolysaccharide in the bloodstream and a leaner body mass index (BMI), which is associated with healthier lipid, bone, and muscle metabolism [5] [1] [3]. Active persons have more proteinaceous resistance to heat shock and breakdown of epithelial junctions as well, resulting in a more highly adapted and resilient gut barrier, in turn protecting against contact between the immune system and microbial populations [5] [1]. This protection is needed due to the fact that the gut-associated lymphoid tissue in the large and small intestine contains roughly 70% of the body’s immune cells, which produce necessary anti-microbials to guard against unwanted agents and maintain homeostasis between microbes and host [1]. However, these anti-microbial factors are damaging to the gut microbiota, and due to their high proximity, an effective barrier is important [1].
A proposed reasoning for why exercise brings about these positive gut changes is that exercise speeds the movement of contents through the gastrointestinal tract through its stimulation of the enteric nervous system. The contents may have harmful components such as carcinogens, and reducing the time of content exposure allows a more diverse gut microbe population to thrive [6] [3]. However, this reasoning is largely based on studies of acute, rather than habitual, exercise [3]. Another reason is the measurable effect of exercise on the environment of the gut and the bacterial composition of gut microbiota. Exercise secondarily impacts the pH of the intestinal environment, and improves microbial nutrition, mucus secretion, and the formation of biofilms [1]. An increase is seen in mucosal bacteria, such as Akkermansia muciniphila, which are the key maintainers of the gut mucosal lining. The gut mucosal lining keeps microbes from permeating the gut epithelium and becoming exposed to the damaging effects of the immune cells and outer environment [1].
Butyrate
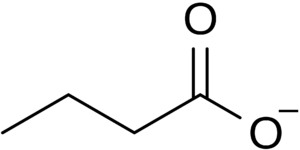
An increase in the compound butyrate is one of the most significant exercise-induced effects seen in the gut microbiome. Butyrate is a type of short-chain fatty acid (SCFA) produced by the fermentation of dietary fiber by the microbes of the large intestine. 30 - 60 % of the bacteria of the colon are the anaerobic gram-positive clostridial clusters IV and XIVa, which perform fermentative metabolism generating butyrate, lactate, and formate [7]
. Butyrate is fuel for colonocytes, or colon epithelial cells [7] [8]. The healthy replacement of colonocytes is necessary for maintaining the gut barrier which also plays a role in preventing the aforementioned permeability of harmful substances
[8] [1]. Butyrate also has an influence on gene expression, cell development, and regulating the host immune system, and a higher measured amount of butyrate correlates with the reduction of IBS symptoms seen from exercise [1] [7].
Two of the most prominent pathways found for the synthesis of butyrate seen in gut bacteria are described as follows: both begin by utilizing the substrate butyryl-CoA which is produced via a process similar to a reverse oxidation of fatty acids. Two molecules of acetyl coenzyme A are reduced into butyryl-CoA, which is formed into butyrylphosphate by the enzymes phosphotransbutyrylase and butyrate kinase in pathway one, or transferred to an external acetate by the enzyme butyryl-CoA:acetate CoA-transferase in pathway two. The first pathway produces ATP alongside butyrylphosphate, and is the pathway more often used in the intestines [7]. The second pathway produces butyrate and reforms acetyl-CoA, and is also performed in the human body, for example by bacteria of the genera Roseburia and Faecalibacterium [7].
After production from the pathways, butyrate is metabolized by the mitochondrial tricarboxylic acid cycle. Cytosolic citrate and acetyl-CoA are produced from the cycle and accumulate, and the speed of protein histone acetylation by the enzyme histone acetyltransferase increases, resulting in higher expression of certain cell replication genes [8] [1]. Alternatively, butyrate has a negative effect on colorectal cancer cells. Rather than being metabolized by mitochondria, butyrate builds up in the cell cytosol and disrupts mitochondrial function. Rather than increasing the speed of histone acetylation, free butyrate becomes an inhibitor of the process. Thus, butyrate performs the opposite role in cancerous cells by suppressing the proliferation of cells. The presence of butyrate leads to greater cell death in these conditions, making it a phenomenal force against colorectal cancer
[8] [1]. Instance of colorectal cancer is negatively correlated with the amount of butyrate-producing bacteria types [1].
Types of butyrate-producing bacteria include F. prausnitzii, R. hominis, Clostridium acetobutylicum, Eubacterium cylindroides, Faecalibacterium prausnitzii, Roseburia hominis, Akkermansia muciniphila, Butyrivibrio fibrisolvens, Coprococcus sp., Eubacterium ruminantium, Faecalibacterium prausnitzii, Clostridium leptum, Eubacterium rectale, Roseburia spp., Eubacterium hallii and Anaerostipes spp. as well as many more possible species of Firmicutes, Actinobacteria, Bacteroidetes, Fusobacteria, Proteobacteria, Spirochaetes, Odoribacter, Rikenellaceae, Thermotogae, Ruminococaccaceae, and Lachnospiraceae [9] [7] [5] [10] [11]
Studies on Exercise and Microbiota
Animal Studies
One of the challenges in evaluating the effect of exercise on the microbiome of any species are the plethora of confounding variables. Things like age, exercise type, exercise history, medication, and countless other factors all affect microbial diversity and abundance [1]. Variance in diet alone can account for huge differences in gut microbiota, down to the ratio of macronutrients or fiber level or calorie intake [1]. Different studies on the effect of exercise on the microbiome of mice have yielded vastly different results due to the inconsistencies in specimen type and condition, and experimental setup. Some studies saw an increase in the firmicute to bacteroidetes ratio in exercised mice, whereas some studies found the opposite or no change at all. Others found significant resulting microbial differences in mice that were subjected to the same exercise, but fed varying levels of fat, or that were of a different age [1]. The more controlled that a study can be, the more accurately a cause-and-effect relationship can be determined. Animal experimental studies are generally easier to control for incongruent variables than human experimental studies.
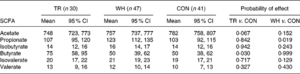
A study by Basterfield and Mathers tested the effects of exercise and corresponding butyrate production on mice with tumorous colons. They measured the fecal butyrate concentrations over 12 weeks of three mice groups – one forced to run on a treadmill 30-60 minutes a day for 5 days a week, one given an exercise wheel to engage in voluntary exercise on, and one unexercised control group. The study found a reduced number and size of large tumors in the colon and a higher mean fecal butyrate concentration in the treadmill group compared to other groups, displaying that exercise and butyrate offer a direct inhibition toward colonic tumorigenesis (Table 1). Curiously, the voluntary exercise group did not see higher butyrate concentrations or reduced tumourosity compared to the control group for reasons that the researchers could not be sure of [12].
Matsumoto et al. also tested the effects of long-term exercise on the microbiota and SCFA composition found in the cecum of young male rats. Rats were first kept sedentary for a week and then split into a sedentary control group and a group allowed to perform “voluntary wheel running” for five weeks. The banding profiles of bacterial DNA extracted from the cecum, determined by PCR-TGGE, were analyzed and found high sequence similarity to butyrate producers SM7/11 T2-87, two strains of Eubacterium cylindroides. Additionally, the cecum of the exercise group was roughly 1.5 times larger and heavier in contents than the exercise group, with the main difference being a higher concentration of butyric acid. Analysis did not show a change in other SCFAs between groups. This study displayed a clear increase in butyrate-producing species caused by exercise [3].
Exercise and associated improvement in the gut microbiome (such as increased butyrate) has been shown to reduce fatty tissue, reduce insulin resistance, and improve metabolism in studies of obese animals [1]. A study by Queipo-Ortuño et al. showed an increased prevalence of beneficial Lactobacillus and Bifidobacterium species in male rats that were allowed six days of voluntary movement on an exercise wheel [13]. An even more explicit correlation between exercise and microbial health is seen in a study by Lai et al. where a fecal transplant from exercised mice resulted in lowered inflammation and fasting blood glucose, as well as weight loss and other improved markers of metabolism, in sedentary mice. The host fecal matter had high levels of butyrate producers Odoribacter and the AF12 strain of Rikenellaceae [11]. Higher amounts of butyrate and other SCFAs generally cause greater satiety hormone production and thus promote more normal eating patterns. These factors, as well as a stronger intestinal wall, correlate butyrate with reduced risk and symptoms of diabetes [1]. Butyrate production, and by extension, exercise, also causes cognitive improvements in animals. Kang et al. tested the effect of exercise in the form of an “hour of daily wheel running” on adult mice [14]. A reduction of anxiety symptoms in the mice was seen alongside an increase in butyrate-producing Lachnospiraceae. Levels of brain-derived neurotrophic factor (BDNF) were increased by butyrate, observed in the rodents’ hippocampi and frontal cortices. BDNF has anti-depressant effects (increase serotonin levels) and is essential for the health of neurons and synapse formation. The BDNF gene is positively regulated by butyrate, and by extent exercise, and is important for neuroplasticity and microglial (brain immune cells) regulation as well [14] [1].
Human Studies
An experiment by Clarke et al. evaluated the microbiomes of elite rugby players, finding that they had greater microbial alpha diversity, or local gut mean diversity, than similarly lean people who did not exercise and people with higher BMI [9]. Fecal and blood samples were evaluated using DNA extraction and PCR amplicon sequencing, and found 11 more distinct phyla in athletes than in the lean group, and 13 more than in the high BMI group. Only a reduction of Bacteroidetes was found in athletes compared to high BMI controls, with greater abundance of 48 other taxa, and a reduction of Lactobacillaceae, Bacteroides, and Lactobacillus with greater abundance of 40 taxa compared to low BMI controls. Both the athletes and the low BMI group had more bacteria of the genus Akkermansia, particularly the aforementioned Akkermansia muciniphila. Persons with obesity and metabolic disorders are often found to have reduced concentrations of Akkermansia muciniphila [9]. The overall body metabolic and inflammatory markers, including measurements of many substances such as glucose, insulin, and plasma creatine kinase levels, were preferable in the athlete group over controls as well. One confounding factor in this study was that the athletes ate a generally healthier diet higher in protein–diet was not controlled for across groups due to the cross-sectional approach (an observational study where data is collected once, as a sort of “snapshot” of a variable [9] [15].
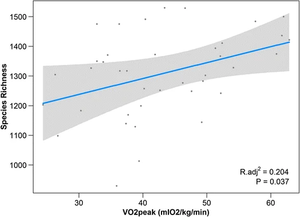
Estaki et al. performed an experiment to determine the relationship between cardiorespiratory fitness and gut microbial composition from fecal samples. 39 young adult subjects controlled for age, BMI, illness, chronic disease, and diet (self-reported) underwent a VO2peak test which measures the highest oxygen consumption at maximal exercise (Figure 1). Researchers found that the measurement of peak oxygen uptake correlated with significant differences in the microbiome. Namely, greater cardiorespiratory fitness was associated with genes for fatty acid biosynthesis, as well as bacterial chemotaxis and motility. Gas chromatography for SCFA analysis found increased fecal butyrate correlated with higher VO2peak, with an increase in prominent butyrate producers Clostridiales, Roseburia, Lachnospiraceae, and Erysipelotrichaceae. Greater overall taxonomic diversity was seen with higher VO2peaks as well [2].
Mailing et al. performed a longitudinal study in which 32 previously sedentary adults of varying BMI (lean BMI < 25 and obese BMI > 30) underwent a 6-week exercise program consisting of three 30-60 minute sessions of endurance activity per week. Diet and other variables were controlled for. They found a decrease of Bacteroides species in lean subjects (comparable to the athletes in Clarke et al.), but surprisingly, an increase of Bacteroides in obese subjects [1]. The opposite trend was seen for the aforementioned Faecalibacterium butyrate-producer. As such, the fecal butyrate, other SCFAs, and acetate concentrations increased in lean subjects [1]. However, the changes mostly reverted back to the pre-exercise levels during the following six-week termed “sedentary washout period” [1]. The implication of these findings is that changes in the microbiota can be temporary and that more research is needed to determine what is necessary to create lasting changes. A proposed reasoning for this finding is that lasting change requires longer implementation of a habit, such as exercise. The following study supports this hypothesis.
A short-term exercise study by Cronin et al., preceding and similarly structured to Mailing et al., tested the effects of three weekly sessions of 18-32 minutes of a mixture of standardized moderate-intensity aerobic training and resistance training on 90 overweight to obese male and female adults, who had been sedentary for three months. Chronic conditions, medication use, antibiotics, bowel preparations, and gastroenteritis were all controlled for. An added testing element was whey protein supplementation, resulting in an exercise group, an exercise and whey group, and a whey-only group. They found increased bacterial diversity in both exercise groups, but a limited change in other aspects, including microbial metabolism. The variance of BMIs (22 - 35 kg/m2) and self-reported diet accountability may have been confounding factors, however, the overall results were not highly significant, again suggesting that longer-term lifestyle interventions may be needed to see significant change. The juvenile development period has been shown to be highly influential on the adult microbiome, and as a result, exercise during childhood more easily creates a more permanent effect than if exercise were initiated for the first time in adulthood [6]. Cronin et al. also implemented a relatively less strenuous exercise regime, which could have also explained the minimal results. Clarke et al. (rugby players) and similar studies evaluated elite athletes, those who had performed habitual exercise for many years, and thus found more significant results [9]. Nonetheless, findings suggest that positive microbial change can be achieved by exercise and maintained over time at any age with consistency.
Contradiction on the Benefits of Exercise
There was a surprising reduction of archaea in the exercise-only group in Cronin et al. The hypothesis that exercise can have a deleterious effect on some microorganisms is curious, as the question is raised of whether these effects should be avoided. It has been found that gut ischemia, the insufficient circulation of blood to an organ, can occur due to bodily heat stress induced by exercise, particularly high-intensity [16]. Other recipients of splanchnic circulation (the gastrointestinal tract, liver, spleen, and pancreas) may be affected as well. Damage to the gut tissue can occur upon reoxygenation after ischemia, also known as an ischemia-reperfusion injury. As such, it is true that acute instances of high-impact exercise such as that seen in military training, or highly prolonged exercise such as long-distance running, can cause damage to the gut barrier and microbial composition [6]. However, these impacts were not seen in lower-intensity exercise and were generally impermanent with sufficient recovery. In fact, exercise is what is known as a “hormetic stressor,” providing a level of stress to the body that is actually necessary for better health and long-term improvement.
Conclusion
There is unquestionably a highly positive, direct effect from exercise on the gut microbiome. Research continues on exploring the nuances of this effect, addressing remaining questions of how to obtain the best results through type of exercise, how exercise can complement the beneficial effects of diet on microbiota, and more, as well as how these nuances and requirements shift depending on the countless variations in human life, including age, health, history, medication, and environment. A promising future is in store for a more deep understanding of the gut microbiome and how it might be shaped to cure disease and help people live their healthiest and happiest life.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 Mailing, L. J., Allen, J. M., Buford, T. W., Fields, C. J., & Woods, J. A. (2019). Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exercise and sport sciences reviews, 47(2), 75-85.
- ↑ 2.0 2.1 2.2 Estaki, M., Pither, J., Baumeister, P., Little, J. P., Gill, S. K., Ghosh, S., ... & Gibson, D. L. (2016). Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome, 4(1), 1-13.
- ↑ 3.0 3.1 3.2 3.3 3.4 Matsumoto, M., Inoue, R., Tsukahara, T., Ushida, K., Chiji, H., Matsubara, N., & Hara, H. (2008). Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Bioscience, biotechnology, and biochemistry, 72(2), 572-576.
- ↑ Meissner, M., Lombardo, E., Havinga, R., Tietge, U. J., Kuipers, F., & Groen, A. K. (2011). Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis, 218(2), 323-329.
- ↑ 5.0 5.1 5.2 Bressa, C., Bailén-Andrino, M., Pérez-Santiago, J., González-Soltero, R., Pérez, M., Montalvo-Lominchar, M. G., ... & Larrosa, M. (2017). Differences in gut microbiota profile between women with an active lifestyle and sedentary women. PloS one, 12(2), e0171352.
- ↑ 6.0 6.1 6.2 Cronin, O., Barton, W., Skuse, P., Penney, N. C., Garcia-Perez, I., Murphy, E. F., ... & Shanahan, F. (2018). A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. MSystems, 3(3), e00044-18.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Louis, P., Duncan, S. H., McCrae, S. I., Millar, J., Jackson, M. S., & Flint, H. J. (2004). Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. Journal of bacteriology, 186(7), 2099-2106.
- ↑ 8.0 8.1 8.2 8.3 Donohoe, D. R., Collins, L. B., Wali, A., Bigler, R., Sun, W., & Bultman, S. J. (2012). The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Molecular cell, 48(4), 612-626.
- ↑ 9.0 9.1 9.2 9.3 9.4 Clarke, S. F., Murphy, E. F., O'Sullivan, O., Lucey, A. J., Humphreys, M., Hogan, A., ... & Cotter, P. D. (2014). Exercise and associated dietary extremes impact on gut microbial diversity. Gut, 63(12), 1913-1920.
- ↑ Parada Venegas, D., De la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., ... & Hermoso, M. A. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in immunology, 277.
- ↑ 11.0 11.1 Lai, Z. L., Tseng, C. H., Ho, H. J., Cheung, C. K., Lin, J. Y., Chen, Y. J., ... & Wu, C. Y. (2018). Fecal microbiota transplantation confers beneficial metabolic effects of diet and exercise on diet-induced obese mice. Scientific reports, 8(1), 1-11.
- ↑ 12.0 12.1 Basterfield, L., & Mathers, J. C. (2010). Intestinal tumours, colonic butyrate and sleep in exercised Min mice. British journal of nutrition, 104(3), 355-363.
- ↑ Queipo-Ortuño, M. I., Seoane, L. M., Murri, M., Pardo, M., Gomez-Zumaquero, J. M., Cardona, F., ... & Tinahones, F. J. (2013). Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PloS one, 8(5), e65465.
- ↑ 14.0 14.1 Kang, S. S., Jeraldo, P. R., Kurti, A., Miller, M. E. B., Cook, M. D., Whitlock, K., ... & Fryer, J. D. (2014). Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Molecular neurodegeneration, 9(1), 1-12.
- ↑ Simkus, J. (2021, Dec 22). How Does the Cross-Sectional Research Method Work?. Simply Psychology.
- ↑ Dokladny, K., Moseley, P. L., & Ma, T. Y. (2006). Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. American Journal of Physiology-Gastrointestinal and Liver Physiology, 290(2), G204-G212.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College
