Lactobacillus jensenii
A Microbial Biorealm page on the genus Lactobacillus jensenii
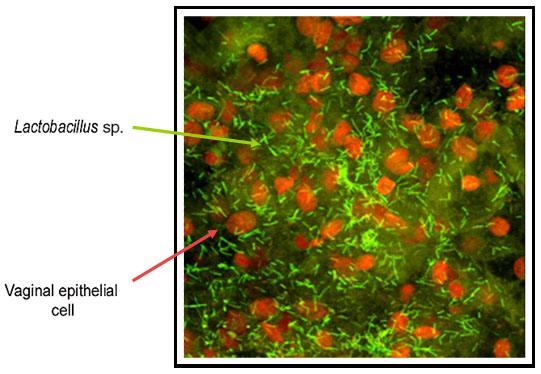
Classification
Higher order taxa
Bacteria; Firmicutes; Bacilli; Lactobacillales; Lactobacillaceae; Lactobacillus
Genus
Lactobacillus jensenii
|
NCBI: Taxonomy |
Description and significance
From the genus Lactobacillus, Lactobacillus jensenii is an anaerobic, Gram-positive bacteria. Its thick peptidoglycan wall gives this non-spore forming bacteria a rod-shaped feature (7). It is one of the predominant species (along with Lactobacillus crispatus) found in the female lower genital tract. It was first discovered while scientists (April 1970) were using gel electrophoresis to do a comparative analysis of lactic dehydrogenase activity in Lactobacillus lichmannii from human vaginal discharge. Although both organisms are phenotypically similar, the DNA composition of this new isolate (36.1 ± 1.2 moles % guanine + cytosine) significantly differed from L. lichmannii in its DNA characteristics (50.8 ± 0.5 moles % guanine and cytosine)(10). Its ability to colonize and be a part of the natural vaginal microflora has recently become a topic of interest to researchers studying prevention of STDs and harmful vaginal bacteria.

HIV/AIDS researchers who are looking for a genetically modifiable organism that would express HIV inhibitors (CV-N) find L. jensenii to be of special interest. So, to ensure there would be sufficient expression of CV-N by this bacterium, the genome of L. jensenii strain 1153 has been partially sequenced. (See Current Research for more details).
Also, because of L. jensenii is a catalase-negative anaerobic bacteria, studies that include L. jensenii anaerobic mechanisms of producing hydrogen peroxide are hot topics for modern researchers who correlate the high colonization of L. jensenii to low colonizations of vaginal bacteria. (See Ecology for more details)
Genome structure
As of June 2007, the genome of L. jensenii 1153 has been fully sequenced using shotgun sequencing by the Lawrence Berkeley National Lab and Osel: The Bacterio-Therapeutics Company, but the data has to be yet released to public (4). For this reason, information on native plasmids and the genome is not available. The genome was sequenced for research conducted at Stanford University, Department of Medicine, that required a better understanding of gene regulation and promoter regions so to increase efficiency of HIV inhibitor cyanovirin-N expression in Lactobacillus jensenii. (See under Current Research for more details)
Most of the sequencing methods include bacterial colony-based strain typing using PCR-fingerprinting and phylogenetic analysis of the partial 16S rRNA gene. Analysis shows that its genome contains over 1600 ORFs which include "novel cell wall anchor domains, unique signal sequences, powerful promoter elements, and possible sites for chromosomal integration of heterologous genes"(9). Its DNA has low G+C content (36.1 ± 2.3 moles % guanine + cytosine), similar to the DNA composition of L. acidophilus - L. jugurti group (36.1 ± 1.2 moles % guanine + cytosine); it produces D-lactic acid as its major metabolic product (10).
Cell structure and metabolism
L. jensenii, like other members of the Lactobacillus, are rod-shaped, anaerobic Gram-positive microorganisms. Ideal growth conditions require the presence of folic acid, vitamin B12, nictonic acid, and calcium pantothenate at 45ºC. It can be differentiated from other species because of the following characteristics: hydrolysis of arginine, production of D-lactate (only), and fermentation of galactose, salicin, esculin, maltose amygdalin, sucrose, ribose, and cellobiose, but not of lactose, melexitose, mannitol, sorbitol, arabinose, and xylose (10).
As an anaerobe and the member of Lactobacillus - bacteria known for acids production, L. jensenii is responsible for producing acid in the vagina by anaerobic metabolism of glycogen. The glycogen is deposited in the vaginal epithelium cells when there is a high level of estrogen, specifically during menstruation period. The vaginal flora and the surrounding epithelial cells metabolize the glycogen into acetic and lactic acid so that the vaginal pH becomes about 4. The strong concentration of the acidic environment help protect the vagina from harmful pathogens in the environment (1).
Ecology
Lactobacillus jensenii has difficulty replicating outside the body. As a matter of fact, L. jensenii (along with Lactobacillus crispatus and Lactobacillus gasseri) is a natural vaginal strain of bacteria colonizing biofilms of the vaginal mucosa in healthy females. This particular vaginal lactobacillus contributes to 23% of the vaginal colony population (following Lactobacillus crispatus-32%), making it the second most prevalent vaginal lactobillus in the lower female genital tract. This hydrogen peroxide producing bacteria contributes to inhibiting anaerobic growth products such as superoxide anion radicals and hydroxyl radicals. Combined with an acidic environment of pH less than 4.5, Bacterial Vaginosis (BV)'s growth as well as transmission and infections of genital related diseases (gonorrhea, urinary tract infection, and chlamydia) or STDs (HIV) are effectively inhibited. Since semen does decrease the acidic environment of the vagina, studies show that repeated and unprotected intercourse increase risk of BV colonization (2).
Pathology
As of June 2007, pathogenicity of Lactobaciluus jensenii for both men and women is unknown. Rather, because of its known role to decrease risks of STDs, this natural member of the human vaginal microflora is considered a protector for the host from acquisition of diseases. (See Ecology for more details)
Application to Biotechnology
Research by Beckman and Ames (1998) demonstrated that free radicals and reactive oxygen species (ROS) help prevent atherosclerosis, diabetes, and cancer. These radicals that form from aerobic respiration during food digestion can damage cells. Food containing antioxidants are able to help quicken the restoration of damaged tissues/cells, therefore, increasing the defense mechanism of the body. Because anaerobic bacteria such as L. jengenii possess anti-oxidant activity, their ability to break down superoxide anion and hydrogen peroxide with milk proteins is the highlight of current studies. Researches are concluding "Lactobacillus jensenii strain showed high antioxidant potential...and it had also high content of hydrophobic amino acids, valine, leucine, phenylalanine and tryptophan." The strain's specific amino acid content matters because the hydrophobic properties of its composition are related to the inhibition of peroxidation activities. Because of this property, we may be able to create fermented milk that can now possess higher antioxidant activity (8).
Current Research
Some of the recent research on Lactobacillus jensenii:
"Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4" (3)
Knowing that HIV enters through cervico-vaginal mucosa, Lactobacillus jensenii, which is known to naturally colonize in the vaginal area, is genetically engineered to secrete two-domain CD4 (2D CD4) proteins. The secreted 2D CD4 binds to anti-CD4 receptor and bound HIV-1 gp120, which shows that the engineering did not affect the L. jensenii proteins structure. Moreover, with the usage of luciferase reproter gene, single-cycle infection assays confirmed that the modified L. jensenii is able to inhibit HIV-1 from infecting target cells. Furthermore, when the bacteria were incubated with recombinant HIV-1HxB2 reporter virus, the infectivity of HeLa cells expressing CD4–CXCR4–CCR5 decreased considerably. The decrease in infectivity of HIV-1 with dosage controlled engineered L. jensenii may be a convenient contraception by using natural occurring colonies of bacteria inside the body to block the transmission of HIV.
Engineered Vaginal Lactobacillus Strain for Mucosal Delivery of the Human Immunodeficiency Virus Inhibitor Cyanovirin-N (9)
Lactobacillus jensenii has been genetically engineered to produce and secrete peptide (CV-N) that prevents fusion with HIV using S-protein layer secretion pathway. The modification did not affect the phenotype of the bacteria, but rather was integrated stably into the chromosome. Genetically engineered L. jensenii was able to proliferate in the vaginas of estrus phase mice as well as secrete this anti-HIV peptide. The results of this new Lactobacillus CV-N hybrid show that it is capable of inhibiting "CCR5-tropic HIVBaL infectivity in vitro with a 50% inhibitory concentration of 0.3 nM". This may be an inexpensive and convenient way to block HIV infection and/or replication in women.
Inhibition of Neisseria gonorrhoeae by Lactobacillus Species That Are Commonly Isolated from the Female Genital Tract (6)
Four strains (Lactobacillus jensenii, Lactobacillus crispatus, Lactobacillus acidophilus, and Lactobacillus gasseri) of Lactobacillus were studied because of past reports of the possible link between high colonization of Lactobacillus and low risk of gonorrhea. In this experiment, Neisseria species or Escherichia coli strains serving as target organisms and four clinical isolates of N. gonorrhoeae were added at different pH on plates containing heart infusion agar and saline solutions of respective Lactobacillus strains. All four strains were able to inhibit the gonococcal strains at low pH while L. jensenii and L. crispatus was also able to inhibit N. gonnorrhoeae at neutral pH. None of them were able to inhibit E. coli strains, though L. jensenii was able to inhibit Neisseria cinerea. On the quantitative scale of the assay, L. jensenii were particularly most resistant to N. gonorrhoeae in the four strains. The strains were able to use hydrogen peroxide as mediator to resist the infection. The paper was able to conclude that the presence of L. jensenii and L. crispatus helps reduce risk of gonorrhea.
References
(2.)Kim A. Brogden, Janet M. Guthmiller. "Polymicrobial Diseases". ASM Press. 2002.
(10.) F. Gasser, M. Mandel, M. Rogosa. "Lactobacillus jensenii sp. nov., a New Representative of the subgenus Thermobacterium. 'Journal of General Microbiology'. 1970 August; Vol 62(2): 219-22.
Edited by Frances Cho, student of Rachel Larsen at UCSD.
KMG