Mechanism of Antibiotic Treatment in Legionnaire's Disease
Introduction
By Jonathan Pang
Legionella (Figure 1) is a genus of rod-shaped, gram-negative bacteria that inhabit fresh-water environments, with the exception of one species (Legionella longbeachae), which resides in soil. Legionella have been shown to inhabit about 80% of freshwater environments, but generally only cause disease in situations where their growth rate has been enhanced by elevated temperatures[1] Legionella are not free-living bacteria. Instead, they are able to survive in freshwater and soil environments by forming commensal or parasitic relationships with protozoa. Legionella replicate intracellularly, and are thus provided with some protection from biocides, antibiotics, pH changes, changes in osmotic pressure, and thermal stress. Apart from ciliated protozoa, Legionella, is also able to replicate in some species of amoebae and slime mold. There is no evidence of Legionella capable of growing without a host outside of on laboratory media. In humans, Legionella infects monocytes, polymorphonucelar leukocytes, and alveolar macrophages [2]. When the human respiratory system is infected by Legionella, it is generally referred to as Legionellosis. Legionellosis can present clinically in three distinct ways. First, it can present as Pontiac Fever, which is a self-limited flu-like illness. Second, a person infected with Legionella can be completely asymptomatic. Last, Legionellosis can present as Legionnaires’ Disease, an illness that affects several organ systems, and is often characterized by pneumonia. There are approximately 50 species of Legionella, but roughly 90% of all cases of Legionellosis are caused by a single species, L. pneumophila. The genus Legionella contains 70 serogroups, and the species L. pneumophila is made up of 15 serogroups. However, L. pneumophila serogroup 1 is responsible for 84% of all cases of Legionellosis. Even though L. pneumophila serogroup 1 only makes up about 28% of environmental Legionella, it accounts for 95% of all Legionella isolated in clinical settings[3]
Pathogenesis
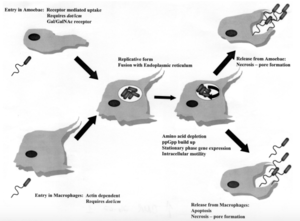
The mechanisms of entrance, replication, and release for Legionella are essentially the same for both protozoa and human macrophages, but there are some key differences (Figure 2). In both cell types, Legionella enter the host cell either by coiling phagocytosis or conventional phagocytosis. In coiling phagocytosis, temporary cytoplasmic projections known as pseudopods begin to engulf the bacterium from opposite ends of the bacterial cell membrane. These pseudopods come to span the circumference of the Legionella cell until it is eventually engulfed[4]. Typically, when this phagocytosis occurs, the engulfed microbe is digested by means of the endocytic pathway. During this process, lysosome-associated membrane glycoproteins, cathepsin D, and other acid hydrolases are recruited and function in phagolysosome formation. L. pneumophila-containing vacuoles (LCVs) delay the onset of these proteins while also recruiting ER vesicles[5]. This takes place within 5 minutes of initial phagocytosis. After approximately 15 minutes, the membrane of the LCV becomes thinner, thus the vacuole begins to resemble the ribosome-studded ER vesicles that previously became closely associated with its membrane. At this time, Legionella converts from its stationary phase to a replicative phase, which is more resistant to the acidity of a lysosomal environment. It stops expressing the factors that delayed the endocytic pathway, which allows the LCV to merge with lysosomes. Legionella then begins to replicate with the aid of nutrients and amino acids provided by the lysosomes[6]. Once the amino acids of the host cell have been depleted, Legionella exits the host by causing pore formation, leading to necrosis or apoptosis. Importantly, cell death and release in human macrophages and alveolar epithelial cells is a non-productive pathway for Legionella because it does not continue the spread of the bacteria via person-to-person contact. Alternatively, Legionella that has infected protozoa can be released in vesicles that can continue the transmission of infection to humans if the vesicles become airborne[7].
History
The first strain of Legionella was isolated from guinea pigs in 1943 by military doctor Captain Hugh Tatlock. At the time, Tatlock was attempting to determine the cause of a mysterious rash illness among soldiers at Fort Bragg, North Carolina. To investigate this, Tatlock inoculated guinea pigs with blood from the afflicted soldiers, and he was able to identify, among other things, a strain of bacteria that would eventually become known as Legionella micdadei. Tatlock ultimately concluded that this bacteria was not responsible for the illness experienced by the soldiers, and determined that the illness was instead caused by a strain of Leptospira[8]. It is still unclear whether the soldiers suffered from leptospirosis alone or if some were also infected with Legionellosis, or more specifically, Legionnaires’ Disease. Other strains of Legionella were isolated in 1947 and 1954, but Legionella was not established as a genus until the 1979, after an outbreak of pneumonia caused by Legionella pneumophila. It is largely believed that the increase in frequency of Legionnaire’s Disease can be attributed to advances in human technology during the first half of the 20th century. Legionella most commonly grow in freshwater environments, and their optimal growth temperature is approximately 35°C[9]. Most naturally occurring freshwater sources do not maintain this optimal temperature. However, there are some human-made freshwater sources that are kept at this high temperature, making them an ideal environment for Legionella to multiply rapidly. For this reason, Legionnaires’ Disease outbreaks have been attributed to cooling towers of air conditioning units, as well as the water heating systems in whirlpool spas. Additionally, potential environments for rapid multiplication of Legionella include some construction zones, particularly those involving the descaling or alteration of plumbing systems[10].
In July of 1976, the 58th annual convention of the American Legion Department of Pennsylvania took place at the Bellevue-Stratford Hotel in Philadelphia. The hotel was originally built in 1904, but had been extensively renovated over the years. The building’s air conditioning system included two water chillers in the subbasement, which chilled and circulated water to about 60 air conditioning units throughout the hotel. The convention took place from July 21-24[11]. Members of the American Legion who had been exposed to Legionella typically began to show symptoms of the illness one week after the start of the convention, or three days after returning to their homes. There were a total of 182 confirmed cases of Legionellosis, with 147 of those cases requiring hospitalization, and 29 cases (16%) resulting in death. The initial symptoms and earliest signs of Legionnaires’ Disease included fatigue, muscle pain, coughing, and headache. Approximately 20% of patients were reported to have dulled senses and decreased alertness. This was followed by fever, difficulty breathing, abdominal pain, and gastrointestinal distress. Fevers usually reached between 38.9°C and 40.6°C within 3 days of the onset of illness. Decreased blood perfusion to tissues, resulting in circulatory shock, was seen in 50% of patients who died, but was not observed in any patients who eventually recovered. However, upper and lower GI bleeding, impairment of renal function, and mild hypotension were seen in patients with both outcomes. In patients who died as a result of this illness, death occurred an average of 10 days after onset[12]. Many antibiotics were employed in the early days of treatment of this Legionellosis outbreak. Specifically, various physicians prescribed cephalothin in conjunction with a steroid, aminoglycosides, chloramphenicol, ampicillin, penicillin, erythromycin, and tetracycline. Importantly, physicians treating the 1976 outbreak found the macrolide erythromycin to be among the most effective treatments for Legionnaires’ Disease. This initial success in the early fight against this newly relevant disease is part of the reason for the frequency of macrolide use in combatting Legionnaires’ Disease in the modern era[13].
Following the 1976 outbreak, a number of theories were posed for the method of transmission for Legionnaires’ Disease. Potential culprits for the spread of this disease included person-to-person contact, animals, contaminated food, water, or airborne pathogens[14]. Person-to-person contact was ruled out using surveys of Legionnaires and their families, as well as hospital records. There was no clustering of cases among roommates as would be expected if the disease would be caused by exposure to an infected person. Also, there was no strong correlation with infection of family members by Legionnaires who returned home from the convention before demonstrating symptoms of disease. There was also no correlation between likelihood of infection and attendance at particular food events. No restaurant was implicated as having a particularly high number of customers who were infected with Legionellosis. Also the Legionnaires who developed the disease did not seem to share a common water source. There was no correlation between consuming water from the hotel and contraction of Legionnaires’ Disease[15]. The initial investigation of Legionnaires’ DIsease in 1976 was ultimately inconclusive in identifying a mode of transmission. Since the 1976 outbreak and subsequent investigation, however, the mode of transmission has been identified as airborne Legionella-containing vesicles produced and released by protozoa in freshwater environments[16].
Research Question: Which Species of Legionella Show Virulence in Human Macrophages
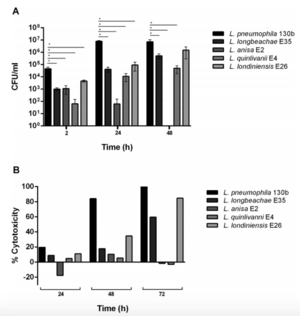
The greatest amount of research concerning life cycle, pathogenesis, and virulence of Legionella has looked at Legionella pneumophila. This is because this species has accounted for 95% of Legionella identified in clinical settings. However, the symptoms associated with Legionella infection (fever, difficulty breathing, abdominal pain, inflammation and accumulation of fluid in lungs) can be attributed to many other diseases. Additionally, Legionella often affects the elderly, smokers, and immunocompromised individuals, so it can be accompanied by other infections that mask Legionella. As a result, some researchers suspect that Legionella other than L. Pneumophila may show virulence in humans, but they have not been discovered because hospitals do not test for them[17]. In order to explore this, researchers collected Legionella quinlivanii, Legionella anisa, L. pneumophila, Legionella londiniensis and L. longbeachae from an urban water source, and tested the ability of these species to infect human macrophages and cause disease (Figure 3). Although L. pneumophila showed the greatest rate of infection and replication, the other species were also shown to be capable of this (Figure 3A). Also, although L. pneumophila showed the greatest percent cytotoxicity, Legionella londiniensis and L. longbeachae also showed some cytotoxicity (Figure 3B). This trait is used as an indicator of ability to cause disease, so it seems likely that Legionella londiniensis and L. longbeachae are virulent in human macrophages. These results suggest that more research should be done to investigate the public health risks posed by these unexplored species of Legionella[18].
Macrolide Mechanism

Macrolides are a class of drug that has been in use as an antibiotic for more than 50 years. They typically function by binding to the bacterial ribosome. The 70S bacterial ribosome is made up of two subunits. The 30S ribosomal subunit binds messenger RNA, while the 50S ribosomal subunit binds tRNA. Inhibiting a necessary subunit of the ribosome prevents the bacteria from synthesizing protein[19]. More specifically, macrolides bind at the peptidyl transferase center of the 50S subunit. This leads to the production of incomplete peptide chains, eventually causing cell death[20]. Macrolides are commonly used in the treatment of infections due to Legionella, Chlamydia, Streptococcus, and Staphylococcus. The first commercially available macrolide for use as an antimicrobial agent was erythromycin, and it continues to be a frequently prescribed treatment for Legionnaires’ Disease despite the production of superior macrolides. Macrolides were initially derived from a species of Streptomyces, and they are among the largest groups of natural products. They are named for the macrocyclic lactone ring that they all have in common, and they are further differentiated according to the number of carbon atoms they contain and their unique sugar moieties[21]. The antibiotic members of the macrolide family typically contain 14-, 15-, or 16-member rings with a number of amino sugars attached (Figure 4). For example, erythromycin can be identified by its 14-member ring with an amino sugar and sugar group attached. A more recently synthesized macrolide is azithromycin. This macrolide has been shown to have superior activity in inhibiting protein synthesis in both gram-positive and gram-negative bacteria. Azithromycin contains a 15-member lactone ring, but it acts upon the same ribosomal subunit that is the target of erythromycin. Azithromycin has been shown to be two to eight-fold more effective than erythromycin in part due to the inclusion of a methyl-substituted nitrogen in its chemical structure. This addition makes azithromycin more resistant to degradation under acidic conditions compared to erythromycin[22].
Another newly introduced group of macrolides are the ketolides. Ketolides contain a 14-member lactone ring, but contain a 3-keto group in place of the C3 L-cladinose group seen in some macrolides. This substitution results in greater acid stability as well as improved antimicrobial activity[23]. Macrolides continue to be an important class of antibiotics for the treatment of respiratory illnesses, like pneumonia.
Fluoroquinolone Mechanism
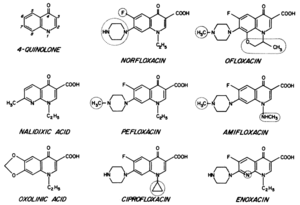
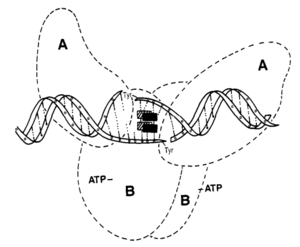
Fluoroquinolones are another class of antibiotic that is used in the treatment some diseases caused by bacteria. The quinolone nucleus is made up of two fused 6-member rings, with these rings containing a nitrogen at the 1-position and a carbonyl at the 4-position (Figure 5)[24]. Quinolones are differentiated according to additional substituents that branch off of this core structure. The quinolones that demonstrate the greatest antimicrobial activity are termed the fluoroquinolones because they all contain a fluorine group. The fluoroquinolones that are of the greatest interest for the treatment of Legionnaires’ Disease are norfloxacin, ofloxacin, ciprofloxacin, amifloxacin, enoxacin, and pefloxacin[25]. In gram-positive bacteria, quinolones act on topoisomerase IV. In gram-negative bacteria, quinolones inhibit the function of DNA gyrase[26]. Because Legionella are gram-negative, the mechanism for inhibition of DNA gyrase is of the greatest interest. The mechanism by which norfloxacin inhibits microbial activity acts as a good example of the antimicrobial function of quinolones.
Norfloxacin inhibits bacterial activity by binding bacterial DNA gyrase. DNA gyrase, or topoisomerase II, is an essential enzyme that functions to relieve tangles and supercoils in double stranded DNA during replication. As strands of DNA are copied, they inevitably overlap and become tangled. DNA gyrase creates a break in one of the tangled strands in order to separate them. The broken strand is then repaired. When DNA gyrase is inhibited by a compound like norfloxacin, tension grows between the tangled strands until one eventually breaks as a result of strain. This type of break is not repaired. Studies have shown that when bacterial cells exposed to norfloxacin die, it is due to an accumulation of lethal DNA breakages[27]. Norfloxacin is a complex-dependent antibiotic. This means that it only binds to DNA gyrase when it has formed a complex with a strand of DNA. DNA gyrase is a tetrameric enzyme. It is made up of two A subunits and two B subunits. The A subunits of DNA gyrase are encoded by gyrA. Mutations to positions 67 through 106 in gyrA sometimes result in resistance to quinolone inhibition. This suggests that quinolones inhibit the activity of DNA gyrase by binding to this region of the enzyme (Figure 6)[28].
Tetracycline Mechanism
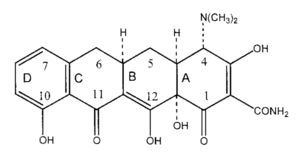
As their name implies, tetracyclines are made up of four linearly fused 6-membered rings with a various unique functional groups attached (Figure 7). Tetracyclines have broad antimicrobial activity, and are used against both gram-negative and gram-positive bacteria. They also have antimicrobial activity against chlamydiae, mycoplasma, rickettsiae, and protozoa. In general, tetracyclines inhibit microbial activity by preventing tRNA from binding to the A-site of the ribosome. This hinders translation, eventually resulting in cell death[29].
Because tetracyclines interact with tRNA and bacterial ribosomes, they must first cross the cell membrane. For gram-negative bacteria, like Legionella, tetracyclines accomplish this by forming a metal ion-tetracycline complex. The most common metal associated with this complex is magnesium. Cationic metal ion-tetracycline complexes can cross Legionella membranes by using channels like the OmpF and OmpC porin channels. Other mechanisms are employed for passage into gram-positive bacteria. Once inside the cell, the most likely target for tetracyclines appears to be the 30S subunit of the ribosome. It is unclear whether tetracyclines bind this site independently or as part of a metal ion-antibiotic complex. Tetracyclines show little activity against eukaryotic cells in part because they do not accumulate in high concentrations within this cell type. Additionally, they show little affinity for the ribosomal subunits of eukaryotes[30].
References
- ↑ Mallegol, J., Fernandes, P., Melano, R. G., & Guyard, C. 2014. Antimicrobial Activity of Solithromycin against Clinical Isolates of Legionella pneumophila Serogroup 1. Antimicrobial Agents and Chemotherapy, 58(2), 909–915.
- ↑ Horwitz, M. A., & Silverstein, S. C. (1980). Legionnaires’ Disease Bacterium (Legionella pneumophila) Multiplies Intracellularly in Human Monocytes. Journal of Clinical Investigation, 66(3), 441–450.
- ↑ Fields, B. S., R. F. Benson, and R. E. Besser. 2002. "Legionella and Legionnaires' Disease: 25 Years of Investigation." Clinical Microbiology Reviews 15.3: 506-26.
- ↑ Horwitz, M. A., & Silverstein, S. C. (1980). Legionnaires’ Disease Bacterium (Legionella pneumophila) Multiplies Intracellularly in Human Monocytes. Journal of Clinical Investigation, 66(3), 441–450.
- ↑ Newton, H. J., Ang, D. K. Y., van Driel, I. R., & Hartland, E. L. (2010). Molecular Pathogenesis of Infections Caused by Legionella pneumophila. Clinical Microbiology Reviews, 23(2), 274–298.
- ↑ Swanson, M. S., B. K. Hammer. (2000). Legionella pneumophila pathogesesis: a fateful journey from amoebae to macrophages. Annu Rev Microbiol. 54: 567-613
- ↑ Fields, B. S., R. F. Benson, and R. E. Besser. 2002. "Legionella and Legionnaires' Disease: 25 Years of Investigation." Clinical Microbiology Reviews 15.3: 506-26.
- ↑ Heuner, Klaus, and Michele S. Swanson. Legionella Molecular Microbiology. Norfolk, UK: Caister Academic, 2008. Print
- ↑ Fields, B. S., R. F. Benson, and R. E. Besser. 2002. "Legionella and Legionnaires' Disease: 25 Years of Investigation." Clinical Microbiology Reviews 15.3: 506-26.
- ↑ Fields, B. S., R. F. Benson, and R. E. Besser. 2002. "Legionella and Legionnaires' Disease: 25 Years of Investigation." Clinical Microbiology Reviews 15.3: 506-26.
- ↑ Fraser, D.W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, et al. (1977). Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 297(22):1189-1197
- ↑ Fraser, D.W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, et al. (1977). Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 297(22):1189-1197
- ↑ Stout, Janet E., Kelly Sens, Sue Mietzner, Asia Obman, and Victor L. Yu. (2004). "Comparative Activity of Quinolones, Macrolides and Ketolides against Legionella Species Using in Vitro Broth Dilution and Intracellular Susceptibility Testing.” International Journal of Antimicrobial Agents 25.4: 302-07
- ↑ Fraser, D.W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, et al. (1977). Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 297(22):1189-1197
- ↑ Fraser, D.W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, et al. (1977). Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 297(22):1189-1197
- ↑ Fields, B. S., R. F. Benson, and R. E. Besser. 2002. "Legionella and Legionnaires' Disease: 25 Years of Investigation." Clinical Microbiology Reviews 15.3: 506-26.
- ↑ Lawrence, Amba, Sofroni Eglezos, and Wlhelmina Huston. (2015). "Environmental Legionella Spp. Collected in Urban Test Sites of South East Queensland, Australia, Are Virulent to Human Macrophages in Vitro." Research in Microbiology 167.2016: 149-53.
- ↑ Lawrence, Amba, Sofroni Eglezos, and Wlhelmina Huston. (2015). "Environmental Legionella Spp. Collected in Urban Test Sites of South East Queensland, Australia, Are Virulent to Human Macrophages in Vitro." Research in Microbiology 167.2016: 149-53.
- ↑ [http://ac.els-cdn.com/S0924857901004010/1-s2.0-S0924857901004010-main.pdf?_tid=d5b01c16-2ddb-11e7-8eb1-00000aab0f01&acdnat=1493580966_a9ba73a43e639dc0230cd4e90256b72e Retsema, J., and Wenchi Fu. (2001) "Macrolides: Structures and Microbial Targets." International Journal of Antimicrobial Agents 18: 3-10. ]
- ↑ Gaynor, M., A. S. Mankin. (2003). Macrolide antibiotics: binding site, mechanism of action, resistance. Curr Top Med Chem. 3(9): 949–961.
- ↑ [http://ac.els-cdn.com/S0924857901004010/1-s2.0-S0924857901004010-main.pdf?_tid=d5b01c16-2ddb-11e7-8eb1-00000aab0f01&acdnat=1493580966_a9ba73a43e639dc0230cd4e90256b72e Retsema, J., and Wenchi Fu. (2001) "Macrolides: Structures and Microbial Targets." International Journal of Antimicrobial Agents 18: 3-10. ]
- ↑ Zhanel G. G., M. Dueck, D. J. Hoban, L. M. Vercaigne, J. M. Embil, A. S. Gin, J. A. Karlowsky. (2001). Review of macrolides and ketolides: focus on respiratory tract infections. Drugs. 61(4): 443-498
- ↑ Zhanel G. G., M. Dueck, D. J. Hoban, L. M. Vercaigne, J. M. Embil, A. S. Gin, J. A. Karlowsky. (2001). Review of macrolides and ketolides: focus on respiratory tract infections. Drugs. 61(4): 443-498
- ↑ Wolfson, J. S., & Hooper, D. C. (1985). The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrobial Agents and Chemotherapy, 28(4), 581–586
- ↑ Wolfson, J. S., & Hooper, D. C. (1985). The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrobial Agents and Chemotherapy, 28(4), 581–586
- ↑ Havlichek, D., Saravolatz, L., & Pohlod, D. (1987). Effect of quinolones and other antimicrobial agents on cell-associated Legionella pneumophila. Antimicrobial Agents and Chemotherapy, 31(10), 1529–1534
- ↑ Shen, L. L., W. E. Kohlbrenner, D. Weigl, J. Baranowski. (1989). Mechanism of quinolone inhibition of DNA gyrase. Appearance of unique norfloxacin binding sites in enzyme-DNA complexes. J Biol Chem. 1; 264(5): 2973–2978
- ↑ Ruiz, J. (2003). Mechanisms of resistance to quinolones: target alterations, decreased accumulation and DNA gyrase protection. Journal of Antimicrobial Chemotherapy. 51: 1109-1117
- ↑ Chopra, I., & Roberts, M. (2001). Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiology and Molecular Biology Reviews, 65(2), 232–260
- ↑ Chopra, I., & Roberts, M. (2001). Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiology and Molecular Biology Reviews, 65(2), 232–260
<ref> Fields, B. S., R. F. Benson, and R. E. Besser. 2002. "Legionella and Legionnaires' Disease: 25 Years of Investigation." Clinical Microbiology Reviews 15.3: 506-26.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
