Microbiome and Love
Introduction
By Mark Lang

As free-thinking, decision making individuals, humans often believe that they get the final say in the choices that they make throughout their lives. These choices range from where individuals go to school, what to eat on a given day, and who to take as a perspective reproductive partner. But how many of these choices are actually under your control? It is a question that philosophers have debated for decades, but perhaps modern microbiology may be of help in answering it. Many individuals would be startled to learn that, perhaps, they are making less of their own independent choices and are more or less following along with the choices of the bacteria that inhabit their bodies. While a surprising revelation, it is one that science is starting to confirm to be true!
Commensal and symbiotic bacteria can be found all over and within the human body. While some occupy the skin, hair (members of bacterial species such as Staphylococcus), and mucosa of humans (members of bacterial species such as Streptococcus and Pseudomonas), many delve much further into the body: It is these bacteria that can have the most profound effect on human behaviors [2] [3]. With the human body playing host to over 2000 species of bacteria and 800 or more species occupying the human gut, there are a multitude of ways in which bacteria might be influencing the choices that humans are making [4].
Falling in love is one of the hallmarks of the human experience and is one of the biggest life changes than any individual will go through. Many unique changes occur at this time: Individuals make changes to their life schedules, changing their diet or sleep schedules, moving in together and cohabitating, sharing drinks, food and sleeping arrangements. New individuals enter their lives while others exit, each bringing their own unique set of bacteria, viruses and microbes[5]. Thus, each individual is exposed to plenty of new colonizing opportunities for microbes that did not previously exist with the more solitary lifestyle of a single individual[5].
Within this page, we will discuss the changes that come about within the microbiome as individual organisms search for partners, “fall in love,” and go through periods of separation following relationship ending events. We will also cover how the human bodily microbiome, most specifically the gut microbiome, influences relationship variables such as how one goes about picking a life partner and what traits they are looking for. Finally, we will cover additional influences of the microbiome on romantic relationships, such as how bacteria are shared between individuals and the long-term health implications of these sharing events.
How the Gut Microbiome Influences Finding a Partner
The gut microbiome has large scale implications on behavior that have been well documented in many previous studies [6] [7] [8]. While it might seem that the gut and the microbiome contained within it are largely disconnected from the brain and the decision-making process in humans, that simply is not the case: The enteric nervous system (which includes all the nerves in the gut and, by default, all the bacteria and microbes that are capable of interacting with those nerves) is connected to the brain via the vagus nerve [9]. Thus, there is a direct avenue of information transport going from your gut microbiome all the way up to your brain. This pathway of interconnectedness not only allows for the sharing of information but also the transport of certain metabolites produced by the gut, including major neurotransmitters such as GABA [9]. Therefore, the gut microbiome is directly influencing the functions of the brain via the enteric nervous system and the vagus nerve.
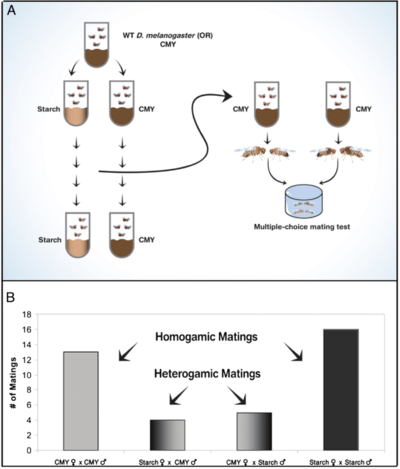
Since there is this extremely strong and powerful connection between the brain and the gut and its microbiota, a logical question would be to ask how the microbes of the gut might influence the decision making that organisms do, especially with regards to important decisions such as finding a mate in order to reproduce and pass on their genetics[10]. While this is a difficult question to ascertain in large organisms such as humans, initial research can be conducted in other organisms to first determine if there is any basis to build future research off of. Thus, several very interesting experiments have been done in a variety of different model organisms in order to test how exactly the gut microbiome influences these important decisions of whom to mate with, when to mate, and whether to abandon one partner or environment for another[10].
Fruit flies, while physically quite different than humans, have a simple set of neurons that are easy to experiment with, along with an enteric nervous system that allows for the observation of gut-brain connections [10].Over the past 15 years, as the important nature of the gut in modulating behavior has become more apparent, research utilizing fruit flies for behavioral purposes related to the enteric nervous system has really taken off[10]. One very complex study stands out in particular for its major findings: Flies would only try to mate with individuals who had a similar gut microbiome to themselves and would show no mating preference towards individuals with dissimilar gut microbiomes [10]. To test this, the researchers reared two different sets of flies on two different types of food; The idea here was that very different food types would lead to very different microbiome colonization within each group [10]. The flies were reared on their set food type from birth, and several successive generations (all the way up to 37) were continued on that specific media so that the microbiome could shift to being optimized for that media, and the researchers confirmed the differences in microbiome composition utilizing fecal microbiota analysis [10]. The researchers then took samples of flies from each of these groups and placed them together to observe mating behaviors, finding that flies would only mate with individuals reared on the same food type as themselves [10]. While this result implies that the flies are selecting only to mate with individuals similar to themselves, it does not explain why they are making those choices; therefore, the researchers, taking another small sample from the overall population of each group, treated the flies with a small dose of broad-spectrum antibiotics in order to kill off their gut microbes [10]. The results of this experiment are more telling, as now, flies displayed no preference for which group they mated with – that is, the removal of the gut microbes removed any instance of mating preference within the sample, adding evidence to the claim that the composition of the gut microbiome might influence who individuals decide to mate with [10]. While the exact mechanisms of how the microbes and their differences between groups were influencing the choices in who to mate with are not fully determined, the researchers hypothesized that the preference changes were due to the gut bacteria influence sex hormones to target individuals similar to themselves [10]. The drastic changes in mating preference between the two groups when not treated with antibiotics compared to after treatment definitely seems to imply that there is some sort of influence coming from the bacterial species that occupy the fruit fly gut [10]. This influence, if it holds true for other species, would profoundly alter what humans tend to view as “free will” in their mating behaviors, and would be of special interest due to the large amount of antibiotics commonly prescribed!
How the Microbiome Changes for Partners
As already evident, the gut microbiome seems to play a role in determining who an organism ends up choosing as a partner[10]. But then, what, if anything, happens to the gut microbiome and its composition once partners get together? Here, finally, there is research that is directly focused on humans and other primates, and the results are truly astounding[11].
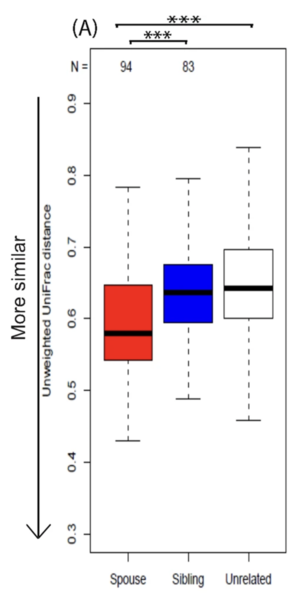
When one thinks of intimate relationships, the transfer of bacteria is likely not the first thought that occupies the mind; Yet, intimate relationships (including but not limited to cuddling, kissing, “making out,” and sexual intercourse) are all about the sharing of the bacteria that make up our microbiota [11]. This “Sexchange” of bacteria during sexual intimacy has wide reaching effects on the human microbiome: One study found that over 80 million bacteria are exchanged during a 10 second kissing session [11]. The study also found several other highly interesting results, the first of which being that all couples showed greater than usual bacterial similarity with their partners [11]. Of course, not all of these bacteria that are exchanged are benign or beneficial. Some, including species like N. gonorrhoeae, are sexually transmitted diseases that can have long term negative health effects [13]. While there will be more information related to this idea later, one idea that has to be thought about (and of which there is no evidence one way or the other) is whether the individuals chose partners who had a similar microbiome to them (like seen above with the fruit flies) or if these similarities occurred due to the exchange of bacteria between partners[12]. Another result from the study was that some of the bacteria shared on exist within the partner’s microbiome for a temporary amount of time before dying off, while other species are more persistent, adapting to the local microbiome and becoming a part of the microbial community that is already present [11]. It is these bacteria that, if pathogenic or carrying some sort of plasmid (such as for antibiotic resistance), could potentially be a problem for the individual whose microbiome they are now occupying. The final major result of the study showed that even though partnered individuals had similar bacteria and exchanged bacteria during the 10 second kissing session, the bacteria that they tended to share were not necessarily the ones that they were sharing; rather, the studies’ authors felt that the partners shared these bacteria due to their similar lifestyle and environments [11]. This theory creates some issues though, as one would then expect siblings raised in the same environment to have more similar bacterial colonization that partners might, something that both this study (and another, discussed below) prove to be false [11][12].
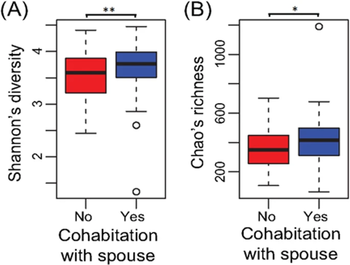
As stated, spouses and partners who live together show a more similar microbiome that do siblings, an interesting finding consider that most siblings are raised together and thus tend to pick up and develop a very similar microbiome due to a nearly identical rearing environment [12]. This study is fascinating as it went beyond just the oral microbiome and kissing to look at how the gut microbiome itself changes due to relationship status [12]. Here the researchers looked at several couples and pairs of siblings, and even included a dietary analysis since the food an individual eats shapes their overall microbiome [12]. After controlling for dietary differences, the researchers found that partnered individuals showed more similar gut microbiomes, just like what was discussed earlier with oral microbiomes [11][12]. Additionally, the study found that partnered individuals had much greater bacterial diversity than those who lived by themselves, and partnered individuals who described their relationships as “extremely close” had both the most similar microbiomes to their partners and the greatest amount of species diversity [12]. This idea, that partners who are extremely close have both a large number of shared bacteria and greater diversity than solitary individuals, offers insight into the idea that having a partner increases an individual’s overall health, something that has been backed up by much of the science on loneliness – however, this is the first instance showing the role the gut microbiome might play in this [12][14]. These findings further support the idea that close, shared relationships work to dynamically alter the microbiome of the participants, with each individual adopting parts of the microbiome of their partner, creating a true connection and unique situation.
A final consideration of how one’s partner might change their microbiome has to address the role of the gut microbiome and its effect on mental health. It is well established that the gut microbiome plays a major role in shaping how individuals respond to various neuropsychiatric disorders, including anxiety and major depression disorders [15] [16]. Thus, if the bacteria that make up the gut microbiome are shared between partners, is it possible that individuals who have microbes that contribute to neuropsychiatric disorders might unwittingly share them with their partners? Could partners who are not afflicted by these neuropsychiatric disorders provide bacteria that counteract those that contribute to these disorders? While the science is not there yet (and neuropsychiatric disorders are more than just bacteria, there are also social, environmental, and genetic factors to include), perhaps in a few years the evidence for these claims might finally exist [17].
Microbiome Changes after Breakup
As established, the gut microbiome seems to influence who individuals take as partners and changes over the course of a relationship to match the microbiome of the partner more closely [10][12]. How then might the ending of a relationship influence the changes that have occurred within the gut microbiome? There is a common saying that a relationship changes who an individual is, with the person being unable to go back to how they were prior to entering a relationship. Is there any merit to that? Might it be possible that these changes in who a person is are driven by the changes in the gut microbiome that the person experienced while in a relationship? And, how permanent are these changes; do they last a few weeks, a few months, the rest of a lifetime? While the behavioral science research is not there to answer questions of this complexity yet, asking these questions and looking into new avenues of exploration are a good start towards tackling these possibilities – ones that many scientists are very interested in [18].
Following the ending of a relationship, there is a period of loss and turmoil due to the many changes that are occurring in the individual’s life, especially if the ending is sudden and without warning [18]. How then might the gut microbiome respond to these rapidly changing conditions and environments[18]? This question was given a great deal of thought by Jie-Yu Chuang, whose seminal paper on the endings of romantic relationships and the changes of the gut microbiome sought to address [18]. As individuals move through the life changing event that is the ending of a relationship, it is likely that the human microbiome is changing in a variety of ways: The loss of a close partner reduces the share of microbes than an individual might be exposed to, the loss of a partner and their social circle decreases the number of microbes an individual might be exposed to, and the increased stress reduces the bacterial diversity of the gut [18]. This last point is especially important, as it is making it more likely for individuals to struggle with other issues at this time of extremely loss, including neuropsychiatric disorders such as depression and greater susceptibility to illness and disease [18]. While there might be ways to prevent these gut changes from occurring (such as by contentiously working to increase the diversity of the gut microbiome via bacterial supplementation or specialty diets), these changes do likely occur, resulting in long term changes for the individuals involved [18]. While the timeline for the changes within the gut microbiome has yet to be fully established, the literature suggests that this might very much be a person specific phenomenon, with some individuals having rapid alterations within their gut microbiome following the loss of a partner, while others may take more time if the microbes are fully established themselves within the microbiome [18]. This would seem to imply that the length of the relationship might also play a role in the amount of time it takes for a microbiome to “revert back” following the loss of a partner, with longer relationships likely resulting in greater bacterial species establishment and thus a longer reversion time (it is important to note here that the microbiome will never fully revert back to how it was before, due to the likely addition of new species, plasmids and metabolic products that might forever change the gut ecosystem) [18]. While much of this remains untested and will likely remain as such for the current time being, it appears that anecdotally there is evidence for many of these claims, and these claims offer an answer as to why the period after the resolution of a relationship leads to such drastic behavioral and physiological changes [18].
Other Effects of the Microbiome on Relationships
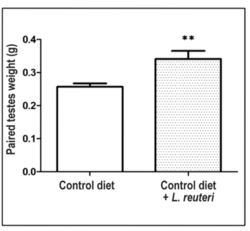
While the role of the human microbiome in shaping romantic relationships and its changes during them has since been established, there are a few other interesting research points that have come out of the field with relation to the changing microbiome. One of the most interesting ones is how the human skin microbiome changes: Men who have more sexual partners have a greater penile bacterial diversity than monogamous men, which would likely also increase their risk of catching some form of sexually transmitted disease [20]. It also seems as though bacterial supplementation, with work done in mice, might have positive increases in sperm count, testicular size, and circulating free testosterone levels, which would likely increase the chances of a male finding a mate and engaging in a successful reproductive event [19]. Both of these studies show how effects of the human microbiome are not just limited to the gut, and they can have far reaching effects on overall behaviors, reproductive events, and live history outcomes [20][19].
Conclusion
While the mechanisms as to how bacterial transfer between partners happens are still under research, as well as how the gut microbiome influences partner selection, the science that does exist on the role of the microbiome in human relationships is quite astounding [5]. Not only does it appear that the microbiome has an effect on whom an individual chooses for a partner, it also adapts to being more similar to that of the partner while in a relationship, and following the conclusion of the relationship is likely left noticeable altered for an unspecified period of time [10][11][18]. It is clear that this field of research still has many potential avenues to pursue, and greater insight into the structural and functional changes of the microbiome occurring both during a relationship and following its conclusion will provide many of these opportunities.
References
- ↑ Jacqueline Quinn, 2017, consciousnessliberty.com
- ↑ Wakeam, E., R. A. Hernandez, D. Rivera Morales, S. R. G. Finlayson, M. Klompas, and M. J. Zinner. "Bacterial ecology of hospital workers' facial hair: a cross-sectional study." Journal of Hospital infection 87, no. 1 (2014): 63-67.
- ↑ Sanderson, Alicia R., Jeff G. Leid, and Darrell Hunsaker. "Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis." The Laryngoscope 116.7 (2006): 1121-1126.
- ↑ Shvets, Yu V., N. Yu Lukianova, and V. F. Chekhun. "Human microbiota and effectiveness of cancer chemotherapy." Exp Oncol 42 (2020): 82-93.
- ↑ 5.0 5.1 5.2 Smith, Leigh K., and Emily F. Wissel. "Microbes and the mind: How bacteria shape affect, neurological processes, cognition, social relationships, development, and pathology." Perspectives on Psychological Science 14, no. 3 (2019): 397-418.
- ↑ Sharon, Gil, Timothy R. Sampson, Daniel H. Geschwind, and Sarkis K. Mazmanian. "The central nervous system and the gut microbiome." Cell 167, no. 4 (2016): 915-932.
- ↑ Ntranos, Achilles, and Patrizia Casaccia. "The microbiome–gut–behavior axis: crosstalk between the gut microbiome and oligodendrocytes modulates behavioral responses." Neurotherapeutics 15, no. 1 (2018): 31-35.
- ↑ Carlson, Alexander L., Kai Xia, M. Andrea Azcarate-Peril, Samuel P. Rosin, Jason P. Fine, Wancen Mu, Jared B. Zopp et al. "Infant gut microbiome composition is associated with non-social fear behavior in a pilot study." Nature communications12, no. 1 (2021): 1-16.
- ↑ 9.0 9.1 Forsythe, Paul, John Bienenstock, and Wolfgang A. Kunze. "Vagal pathways for microbiome-brain-gut axis communication." Microbial endocrinology: the microbiota-gut-brain axis in health and disease (2014): 115-133.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 10.12 10.13 10.14 10.15 Sharon, Gil, et al. "Commensal bacteria play a role in mating preference of Drosophila melanogaster." Proceedings of the National Academy of Sciences 107.46 (2010): 20051-20056.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 Kort, Remco, Martien Caspers, Astrid van de Graaf, Wim van Egmond, Bart Keijser, and Guus Roeselers. "Shaping the oral microbiota through intimate kissing." Microbiome 2, no. 1 (2014): 1-8.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 Dill-McFarland, Kimberly A., Zheng-Zheng Tang, Julia H. Kemis, Robert L. Kerby, Guanhua Chen, Alberto Palloni, Thomas Sorenson, Federico E. Rey, and Pamela Herd. "Close social relationships correlate with human gut microbiota composition." Scientific Reports 9, no. 1 (2019): 1-10.
- ↑ Hook III, Edward W., and Kyle Bernstein. "Kissing, saliva exchange, and transmission of Neisseria gonorrhoeae." The Lancet Infectious Diseases 19, no. 10 (2019): e367-e369.
- ↑ Shankar, Aparna, Anne McMunn, James Banks, and Andrew Steptoe. "Loneliness, social isolation, and behavioral and biological health indicators in older adults." Health Psychology30, no. 4 (2011): 377.
- ↑ Huang, Fei, and Xiaojun Wu. "Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression." Frontiers in cell and developmental biology 9 (2021): 472.
- ↑ Wu, Min, Tian Tian, Qiang Mao, Tao Zou, Chan-juan Zhou, Jing Xie, and Jian-jun Chen. "Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice." Translational psychiatry 10, no. 1 (2020): 1-10.
- ↑ Hankin, Benjamin L. "Adolescent depression: Description, causes, and interventions." Epilepsy & behavior 8, no. 1 (2006): 102-114.
- ↑ 18.00 18.01 18.02 18.03 18.04 18.05 18.06 18.07 18.08 18.09 18.10 Chuang, Jie-Yu. "Romantic Relationship Dissolution, Microbiota, and Fibers." Frontiers in Nutrition 8 (2021): 170.
- ↑ 19.0 19.1 19.2 Poutahidis, Theofilos, Alex Springer, Tatiana Levkovich, Peimin Qi, Bernard J. Varian, Jessica R. Lakritz, Yassin M. Ibrahim, Antonis Chatzigiagkos, Eric J. Alm, and Susan E. Erdman. "Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice." PLoS One 9, no. 1 (2014): e84877..
- ↑ 20.0 20.1 Liu, Cindy M., Bruce A. Hungate, Aaron AR Tobian, Jacques Ravel, Jessica L. Prodger, David Serwadda, Godfrey Kigozi et al. "Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda." MBio 6, no. 3 (2015): e00589-15.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College
