Microbiota of the upper respiratory tract of bottlenose dolphins
Tursiops truncatus, more commonly known as the bottlenose dolphin, is a species of the Delphinidae family [19,23,24]. They are present in nearly all the world’s oceans, in temperate and tropical waters and in bays, sounds, and estuaries [19,23,24].

Ecological significance of dolphins
Dolphins help maintain the marine food cycle by controlling fish populations as top-level predators, preying on old and ill fish [4,24].
Dolphins are marine ecosystem bioindicators, as they are vulnerable to changes in oceans, such as the presence of toxins or declining water quality [4,24]. Bottlenose dolphins concentrate hydrophobic toxins present in marine ecosystem through bioaccumulation in their tissues [4,24]. Toxic compounds such as polychlorinated biphenyls and heavy metal contaminants can reach high concentrations in the blubber of dolphins, and studying dolphins allows us to see the long-term effects of being exposed to these compounds [4,8,24].
The Upper Respiratory Tract
Dolphins breathe through a blowhole
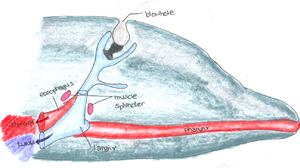
, a unique, specialized nasal opening that prevents intake of water, while permitting the dolphin to inhale and exhale [5,6,12]. A muscular flap covers the blowhole during submergence and, during exhalation, air is rapidly expelled which excludes seawater and thus, the colonization of the blowhole by microorganisms in the ocean is inhibited, as they cannot enter the blowhole [6,14]. An internal barrier also inhibits the exchange of material between the respiratory and gastrointestinal tract, preventing the colonization of the respiratory tract by gastrointestinal microbiota [14]. The blowhole environment is warm, moist, and rich in proteins and lipids since mucous containing microbes from the upper respiratory tract are expelled from the dolphin [6,14]. However, colonization is difficult since the environment is constantly exposed to rapid changes in pressure and is mostly anaerobic with high levels of carbon dioxide [6,14]. Additionally, the upper respiratory tract’s normal flora is kept monitored by the dolphin’s immune system [14]. The dolphin’s immune system inhibits the growth many pathogens that enter the upper respiratory tract through defense mechanisms such as mucociliary linings and secretion of antimicrobial peptides [14].
Microbiota of Upper Respiratory Tract in Bottlenose Dolphins
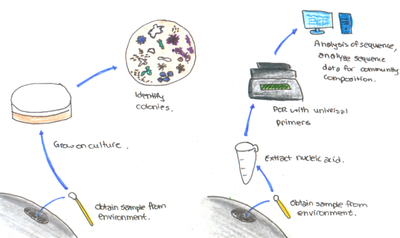
Blowhole samples were subjected to 16S rRNAgene sequencing to determine the presence and structure of microbial communities in the URT [14,17]. Samples from four wild and nine captive dolphins showed high similarities (97% accordance), implying that the unique environment of blowholes select for specialists that are adapted to survive [14,17].
The microbial flora in the URT is substantially novel. Only two of the 1012 recovered sequences had more than 97% similarity to sequencing databases [14].
It was shown that 19 bacterial phyla were present in the bottlenose dolphin’s samples, and 98% of the community composition was made up of only six phyla: the Proteobacteria, Firmicutes, Fusobacteria, Actinobacteria, Bacteroidetes and an unclassified bacterial phylum [14,17]. 96% of all sequences were representative of only three phyla: the Proteobacteria, Bacteroidetes, and Firmicutes, with 1% of the total sequences belonging to Actinobacteria [14,17]. Furthermore, the sequences were found to have the greatest homology with bacteria from the genera Cardiobacteriaceae, Suttonella, Psychrobacter, Tenacibaculum, Fluviicola and Flavobacterium [14,17].
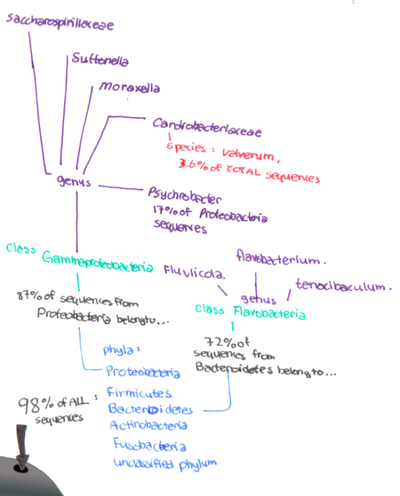
Additional analysis showed that 87% of Proteobacteria sequences belonged to the class Gammaproteobacteria [14]. Sequences within Gammaproteobacteria belonged to the genera Cardiobacteriaceae, Moraxellaceae, Suttonella, and Saccharospirillaceae [14]. An average of 55% of total sequences were from Gammaproteobacteria, with the genera Cardiobacteraceae being most common [14]. 17.1% of the Gammaproteobacteria sequences were from the genera Psychrobacter, which are associated with infections in fish, sheep and humans [10,13,14]. 3.6% of the total sequence was associated with Cardiobacterium valvarum, a bacterium associated with fatal endocarditis in humans [14,17]. Suttonella ornithocola was also found, a bacterium associated with lethal respiratory disease in blue tits (birds) [9,14,16].
72% of the sequences from the phylum Bacteroidetes were from the class Flavobacteria, within the genera Tenacibaculum, Flavobacterium and Fluviicola [3,11,14,15]. Species of Fluviicola and Tenacibaculum are most commonly associated with water, sediment, and soil samples, while Tenacibaculum species are associated with mortality in fishes [11,14,15].
Additional bacterial sequences found include: the Arcobacter and Hydrogenimonaceae (within the Epsilonproteobacteria), Halotalea, Methylococcus, and Marinimicrobium (within the Gammatoproteobacteria) and Aquimarina (Flavobacteria), Helococcus (Clostridia) and Mycetocola (Actinobacteria) [17].
Cardiobacteriaceae
The bacterial family Cardiobacteriaceae (of the class Gammaproteobacteria within the phylum Proteobacteria) consists of facultative, anaerobic, gram-negative rods [7,17,25]. They form part of the normal flora in the respiratory tract of some mammals, but are responsible for opportunistic infections, including endocarditis, bacteremia, and wound infections in humans [7,17]. The high concentration of carbon dioxide facilitates the growth of Cardiobacterium valvarum, leading to their prevalence in the blowhole [7,17].
Psychrobacter
The family Psychrobacter (of the class Gammaproteobacteria) consists of mostly halotolerant, aerobic, non-motile, non-pigmented, non-sporulating, catalase positive, oxidase positive, gram negative coccobacilli, most commonly found in cold aquatic environments [10,13]. This family is closely related to the Moraxella family, also within the class Gammatoproteobacteria [13]. Some Psychrobacter are associated with infections in fish, such as Psychrobacter immobilis, leading to the death of many fish [13]. Psychrobacter were also shown to cause infections in humans, processed meat and poultry as well [10].
Tenacibaculum

Tenacibaculum is a genera within the class Flavobacteria of the phylum Bacteriodetes [11,15,20].Tenacibaculum are generally gram-negative, pale yellow-pigmented bacteria that are rod-shaped [11,15]. Tenacibaculum maritimum causes ulcerative disease (tenacibaculosis), which affects and leads to the death of many fish [11,15]. Tenacibaculum maritimum is a gram-negative, filamentous bacterium [11,15]. It causes lesions, ulcer, necrosis, and mouth erosions, leading to opportunistic infections and ultimately the death of the fish [11,15].
Looking at the different groups of microbes that inhabit the URT, we see that many of them are pathogenic microbes. However, there is no evidence that indicates that the presence of pathogenic organisms in dolphins lead to their death, as the dolphins that were studied were classified as healthy [14,17]. This implies that the microbes in the URT originated from pathogens that were able to colonize the host despite the harsh environment without causing disease [14,17].
Further Research
There is still much to be learned about the microbiota in the URT of dolphins in addition to the microbes that colonize them [14,17]. Studying these microbes would improve our knowledge of host-microbe symbiosis in dolphins, facilitating the development of novel treatments for illnesses in dolphins [4,14,17].
References
1 Amann, R. I., W. Ludwig, and K. H. Schleifer. "Phylogenetic Identification and in Situ Detection of Individual Microbial Cells without Cultivation." Microbiology and Molecular Biology Reviews 59.1 (1995): 143. Print.
2 Barrett, Norman, and Franklin Watts. Dolphins. 1st ed. London, Great Britain: Franklin Watts Ltd, 1989. 10. Print.
3 Bernardet, J. F., et al. “Cutting a Gordian Knot: Emended Classification and Description of the Genus Flavobacterium, Emended Description of the Family Flavobacteriaceae, and Proposal of Flavobacterium Hydatis norn. Nov. (Basonym, Cytophaga Aquatilis Strohl and Tait 1978)." International Journal of Systemic Bacteriology 46.1 (1996): 128. Print.
4 Bossart, Gregory D. "Case Study: Marine Mammals as Sentinel Species for Oceans and Human Health." Oceanography 19.2 (2006)Print.
5 Buck, John D., et al. "Aerobic Microorganisms Associated with Free-Ranging Bottlenose Dolphins in Coastal Gulf of Mexico and Atlantic Ocean Waters." Journal of Wildlife Diseases 42.3 (2006): 536. Print. [6] Coulombe, Harry, Ridgeway, Sam, and Evans, Williams. "Respiratory Water Exchange in Two Species of Porpoise." Science 149 (1965): 86. Print.
7 Das, M., et al. "Infective Endocarditis Caused by HACEK Microorganisms." Annual Review of Medicine 48 (1997): 25. Print.
8 Davison, N. J., et al. "Infection with Brucella Ceti and High Levels of Polychlorinated Biphenyls in Bottlenose Dolphins (Tursiops Truncatus) Stranded in South-West England." Veterinary Record (2011)Print.
9 Foster, Geoffrey, et al. “Suttonella Ornithocola Sp. Nov., from Birds of the Tit Families, and Emended Description of the Genus Suttonella." International Journal of Systemic and Evolutionary Microbiology 55 (2005): 2269. Print.
10 Gini, Gustavo. “Ocular Infection Caused by Psychrobacter Immobilis AcquiredintheHospital." Journal of Clinical Microbiology 28.2 (1990): 400. Print.
11 Herrera, Ruben, Toranzo, Alicia, and Magarinos, Beatriz. “Tenacibaculosis Infection in Marine Fish Caused by Tenacibaculum Maritimum: A Review." Diseases of Aquatic Organisms 71 (2006): 255. Print. [12] Hilty, Markus, et al. "Disordered Microbial Communities in Asthmatic Airways." PLoS ONE 5.1 (2010): e8578. Print.
13 Hisar, Olcayl, Telat Yanik, and Hisar, Sukriye. "Clinical and Pathological Investigation of Psychrobacter Immobilis Infection in Rainbow Trout (Oncorhynchus Mykiss, Walbaum).” the Israeli Journal of Aquaculture 54.4 (2002): 189. Print.
14 Johnson, Wesley R., et al. "Novel Diversity of Bacterial Communities Associated with Bottlenose Dolphin Upper Respiratory Tracts." Environmental Microbiology Reports 1.6 (2009): 555. Print.
15 Jung, SeoYoun, TaeKwang Oh, and JungHoon Yoon. " Tenacibaculum Aestuarii Sp. Nov., Isolated from a Tidal Flat Sediment in Korea." International Journal of Systemic and Evolutionary Microbiology 56 (2006): 1577. Print.
16 Lawson, Becki, et al. " Acute Necrotising Pneumonitis Associated with Suttonella Ornithocola Infection in Tits (Paridae)." The Veterinary Journal 188 (2011): 96. Print.
17 Lima, Nichole, et al. "Temporal Stability and Species Specificity in Bacteria Associated with Bottlenose Dolphins Respiratory System." Environmental Microbiology Reports 4.1 (2012): 89. Print.
18 Ludwig, Wolfgang, Euzeby, Jean, and Whitman, William. "Road Map of the Phyla Bacteroidetes , Spirochaetes , Tenericutes ( Mollicutes ), Acidobacteria , Fibrobacteres , Fusobacteria , Dictyoglomi , Gemmatimonadetes , Lentisphaerae , Verrucomicrobia , Chlamydiae , and Planctomycetes." Bergey's Manual of Systematic Bacteriology. 4 Vol. Print.
19 Moffett, Martha, and Robert Moffett. "Bottlenose Dolphin." Dolphins. United States of America: Franklin Watts Inc, 1971. 23. Print.
20 O'Sullivan, Louise, et al. " Fluviicola Taffensis Gen. Nov., Sp. Nov., a Novel Freshwater Bacterium of the Family Cryomorphaceae in the Phylum ‘Bacteroidetes’." International Journal of Systemic and Evolutionary Microbiology 55 (2005): 2189. Print.
21 "Phylogeny and Protein Signatures for Actinobacteria (High G+C Gram-Positive Bacteria)." Bacterial/Prokaryotic Phylogeny. 03 2007.Web. <http://www.bacterialphylogeny.com/groupspecific/actinobacteria/actinobacteria.html>.
22 "Phylogeny and Protein Signatures for Firmicutes (Low G+C Gram-Positive Bacteria)." Bacterial/Prokaryotic Phylogeny. 04 2007.Web. <http://www.bacterialphylogeny.com/groupspecific/firmicutes/firmicutes.html>.
23 Simon, Seymour. "Habitat." Dolphins. 1st ed. New York, United States of America: HarperCollins Publishers, 2009. 10. Print.
24Wells, Randall S., et al. "Bottlenose Dolphins as Marine Ecosystem Sentinels: Developing a Health Monitoring System." EcoHealth 1 (2004): 246. Print.
25 Williams, Kelly, P., et al. "Phylogeny of Gammaproteobacteria." Journal of Bacteriology 192.9 (2010): 2305-14. Print.
26 Wolf, Matthias, et al. " Phylogeny of Firmicutes with Special Reference to Mycoplasma (Mollicutes) as Inferred from Phosphoglycerate Kinase Amino Acid Sequence Data." International Journal of Systemic and Evolutionary Microbiology 54 (2004): 871. Print.

