Molluscum contagiosum virus
Overview
The first description of the molluscum contagiosum virus was made in 1814. This first written account noted the features of the virus that were distinct from neurofibromatosis, a disease which causes tumors in nervous tissue [2]. Molluscum contagiosum virus itself is a poxvirus. Similar to the smallpox (variola) virus, molluscum contagiosum is a skin disease that is exclusive to humans. However, smallpox was declared to be eradicated in 1980 Cite error: Invalid <ref> tag; invalid names, e.g. too many whereas molluscum contagiosum persists.
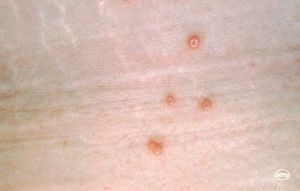
Figure 1 shows the primary symptom of molluscum contagiosum infection: skin pustules. The primary symptom of infection with this virus are round, painless pustules on the skin that range in size from the size of a pinhead to a pencil eraser [3] . The virus itself is 100 by 200 by 300 nm in size [2]. People of all ages can contract this virus but it is most common in children. For adults, it can be considered an sexually transmitted infection because it spreads by skin-to-skin contact. [3]
Infection
In individuals with healthy immune systems, the skin disease caused by molluscum contagiosum infection is usually benign, not severe, and self-limiting [4]. The disease itself is characterized by skin pustules, called Mollusca, that have been found to appear on all parts of the body including the face, abdomen, legs, neck, arms, and genital region. However, they are rarely found on the palms of the hands or soles of the feet. The lesions can either be found in clusters or can be on their own [5]. A typical patient infected with molluscum contagiosum will usually have no more than 20 pustules but in some patients, more than 100 lesions have been found [4].
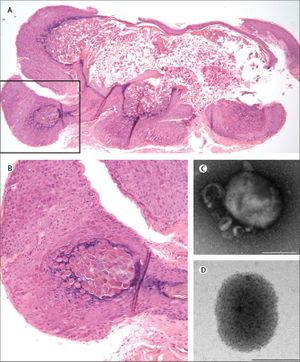
The Mollusca bumps themselves usually present similarly. They are small raised bumps, ranging from two to five millimeters in size and can be white or pink in color. At the center of the bumps there is usually a depression or dimple. They have been described to have a “pearly” appearance. The Mollusca are not typically painful but they can occasionally be itchy, sore, red or swollen [5]. In order to diagnose molluscum contagiosum, doctors will typically just look for the pustules characteristic of the infection. Using a dermatoscope, however, can aid in diagnosis by highlighting vessels and lesional orifices that are characteristic of the pustules. A dermatoscope can be used to examine other vascular features typical of molluscum contagiosum infection as well, such as the crown, punctiform, and radial patterns [4]. Histological characteristics of the pustules can be seen in figure 2. Histopathology helps uncover characteristic qualities of a Mollusca. For example, the lesions will have a single punctum, a small and distinct point. They will also have an increased cellular reproductive rate, which is known as epidermal hyperplasia. Within this buildup of cells lie the molluscum bodies. These bodies develop below the surface of the skin. They progress from deeper to more superficial skin layers. By the time they rise to the top, they are at their largest. Histologically, the characteristics of the molluscum contagiosum infection have been compared to keratoacanthoma. Both skin diseases involve increased cell proliferation, epidermal shouldering at the periphery of the lesion, and a central dimple [6].
The two ways that the virus can spread is from person-to-person skin contact or through contact with fomites. Person-to-person contact involves infected skin touching healthy skin, thus spreading the infection. This also means that the infection can auto-inoculate if the infected person touches infected skin to uninfected skin. Often, this happens through touching or scratching lesions and then touching another part of the body. Mollsucum contagiosum is often considered a child’s disease. However it also can appear in adults, where one of the most common ways to spread this infection to others is through sexual contact. It has not been clarified whether the disease can spread when the Mollusca are intact or if the lesion must be broken in order for transmission to occur. The other method of transmission is through fomites. Fomites are objects like clothes, towels, bathing sponges, pool equipment, or toys that can carry the virus and spread it. This demonstrates that the virus does not need a human host to survive. Additionally, it is important to emphasize that the virus is localized to the top layers of skin and thus does not get into the bloodstream or to other internal parts of the body. Therefore, it is not possible to transmit the infection through coughing or sneezing [7].
As stated previously, children – particularly those between the ages of 1 and 10 – are the most commonly infected group. Transmission of the infection for children is typically through general contact and through contact with molluscum contagiosum-infected fomites. With adults, the infection is often spread through sexual contact. There are several risk factors that increase the likelihood of contracting the infection. Molluscum contagiosum is an opportunistic infection. Thus, immunocompromised individuals, such as those with HIV, are more susceptible. Additionally, the symptoms of infection can be worse for these individuals. The lesions can be larger and more difficult to treat. Another risk factor is atopic dermatitis, which is a condition that presents as a rash and can cause breaks in the skin. Because of these skin breaks, it is easier for the infection to spread to other parts of the body. Another suggested risk factor is living in a warm, humid climate or places that are crowded. However this has not been definitively proven. On the whole, documented cases of molluscum contagiosum seem to have been on the rise since 1996. This is difficult to confirm because the infection can resolve itself and is infrequently monitored [8].
While treatment is not always necessary for molluscum contagiosum infection, as it can often resolve itself, it is sometimes recommended in circumstances such as infection of the genital region. There are three overall categories of treatment – physical removal, oral therapy, and topical therapy. Physical removal can involve the use of liquid nitrogen to freeze off the lesion (a technique known as cryotherapy), piercing the pustule and extracting the inner “cheesy” substance (curettage), or laser therapy [9]. Though these techniques effectively remove the pustules, the disadvantages are pain, inconvenience, hyperpigmentation, hypopigmentation, and scarring [4]. Oral therapy is a less immediate and invasive form of pustule removal. Oral therapy is often recommended for younger children as it avoids a more painful and frightening procedure. One of the most common oral therapies is cimetidine. Cimetidine is a safe, painless, and well tolerated drug. One downside, however, is that this drug is not as effective in resolving facial lesions as it is in resolving lesions on other parts of the body [10]. The third treatment option is topical therapies. One possible topical therapy is podophyllotoxin cream (0.5%) which can be directly applied to the pustules. However, it is not recommended for pregnant women as it is presumed to be toxic to fetuses. Other options include salicylic acid, potassium hydroxide, tretinoin, cantharidin, and imiquimod. Typical side effects of topical treatments include blistering, erythema, pruritus, stinging, mild dermatitis, and dyspigmentation [4]. Of these topical treatments, cantharidin is among the most effective and well-tolerated. It works as a phosphodiesterase inhibitor. It does often result in a small blister on the skin but because the blister is so superficial, it rarely leaves a scar. It is thought that cantharidin initiates an immune response at the site of the pustule which leads to resolution [11]. While the immune system can often resolve the pustules, another method of self resolution is physical damage or trauma. The pustules are very susceptible to physical damage which typically means scratching or picking. However, this can also result in further auto-inoculation [2].
There are no molluscum contagiosum infection-specific drugs. This is due to the fact that the virus does not replicate well in cell culture. Guan et al. (2014), however, identified a potential drug target in the virus called mD4. In vitro, mD4 is important polymerization during DNA synthesis. The researchers of this study found a small compound that bound to mD4 that inhibited processive DNA synthesis in vitro. They then used the vaccina virus, which is very similar genetically to the molluscum contagiosum virus, to make a hybrid vaccinia virus, mD4-VV. Instead of the vaccina D4 gene, this hybrid virus had a gene that targeted mD4, and thus needed this compound to grow in culture. These findings could help both uncover a way to grow the molluscum contagiosum virus in culture and then potentially develop a virus-specific treatment or therapeutic drug [12].
There are a variety of preventative measures that can reduce the risk of spreading molluscum contagiosum infection. Most of them involve hygienic practices such as hand washing, avoiding touching lesions, covering lesions, not sharing clothes, towels or any other potential fomites, and not swimming [13].
The symptoms of molluscum contagiosum can be mistaken as symptoms of other infections. For this reason, it is important to consider the possibility of cryptococcosis, basal cell carcinoma, keratoacanthoma, histoplasmosis, coccidiomycosis, and verruca vulgaris when attempting to diagnose molluscum contagiosum. Additionally, if pustules are found in the genital area, diagnosis with condyloma acuminata and vaginal syringomas should also be considered possibilities [10].
Characterization and Classification
The poxvirdae family of viruses – of which molluscum contagiosum virus is a member – are viruses characterized by their large size and their double-stranded DNA. More specifically, molluscum contagiosum is a member of the Chordopoxvirinae subfamily [2]. Molluscum contagiosum and the vaccina virus, which served as the smallpox vaccine and a member of the orthopoxvirus genus, have many similar features. However, the vaccina virus has a much more guanine and cytosine [14].
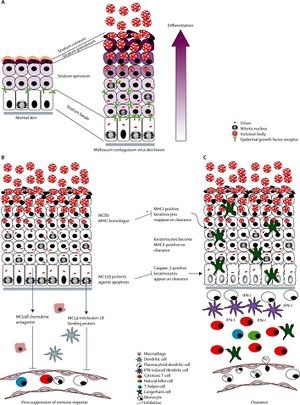
The overall appearance of the virus is rectangular. The core of the virus is sort of cinched and the center to give an overall “dumbell-shaped” appearance. The outer membrane itself is composed of a lipoprotein bilayer [2]. Overall, the virus’s genome consists of 182 genes, of which 154 are likely coding genes [4]. One of the primary characteristics that distinguishes molluscum contagiosum from other poxviruses is that it only replicates in the human epidermis. The smallpox virus also has this characteristic, but it has been eradicated. Once situated in the epidermis, the molluscum contagiosum virus enhances cell mitosis but also disrupts epidermal differentiation. It is not entirely clear how the virus is able to upregulate epidermal cell mitosis because the virus itself cannot make its own epidermal growth factor. However, upregulation of epidermal growth factor expression is found in epidermis infected with molluscum contagiosum [4]. Further, the virus has been found to have increased mitosis and transmit time as well as prolonged survival of infected cells in the follicular epithelium [4]. The lifecycle and local immune response to molluscum contagiosum virus can be seen in Figure 3.
The characteristic pustules of molluscum contagiosum infection have several distinct features. They are small lobes are made up of skin tissue cells, in this case, squamous epithelium, that has over-proliferated. Within the pustules are keratin fragments, which are a fibrous structural proteins that protect epithelial cells from stress or damage, and Henderson-Paterson bodies, inclusion bodies within the cytoplasm which are a collection of proteins [4].
Over time, four subtypes (I, II, III, IV) of molluscum contagiosum have been differentiated. These types are not distinguishable by the pustules they induce. Rather, they are recognized by differences in nucleotide sequences. The most common types are I and IV. The next most common is type II, which is usually found in HIV patients [4]. None of the types have been found to preferentially localize on any part of the body. Additionally, one patient will usually only exhibit only one of the four types, though mixed infections have been documented [2].
One study looked for trends – in type and distribution – of molluscum contagiosum infection in children. Over 50% of the participants in this study demonstrated pustules on more than one part of their bodies. Additionally, concomitant infection with atopic dermatitis was common, affecting 24.2% of patients studied. It was also found that one of the patients was immunosuppressed, as evidenced by low levels of immunoglobulin E (IgE). Overall, no relationship between gender and infection rate was found [15].
Because molluscum contagiosum does not replicate in vitro, it is difficult to determine its replication cycle. However, some elements of its cycle are understood. Like all poxviruses, molluscum contagiosum replicates cytoplasmically. First, just the viral cores are found in the basal layer of the epidermis. Their presence activates keratinocytes and expression of epidermal growth factor receptor increases substantially. As they move upward through the skin, they become Henderson-Paterson, or viral inclusion, bodies. Towards the granular layer, they become larger and larger and can act as an assembly site to make more copies of the virus. The growth and assembly of the virus displaces cellular organelles such as the nucleus. Overall, it takes five days for the virus to fully mature. As the Henderson-Paterson bodies grow, the stratum horneum, which is the outer layer of skin, disintegrates, and thus the virus can spread further [4].
Defense against apoptosis
Molluscum contagiosum virus lacks many of of the molecules that other poxviruses have that help defend against the host’s immune system. It does, however, have 70 open reading frames, that code for components of many different defenses against the immune system. Such defenses include a homolog of cellular chemokine, a selenocysteine-containing gluthione peroxidase, and a death effector domain protein. All three of these defenses are involved in inhibiting apoptosis, which allows the molluscum contagiosum virus to continue replication in the skin [16].
Apoptosis is a highly regulated form of programmed cell death. Caspases (cysteine-aspartate-specific enzymes) degrade cellular components and, when activated, induce apoptosis in order to minimize harm to surrounding cells or tissue. One of the pathways that leads to apoptosis occurs when certain ligands attach to Fas, or another member of the tumor necrosis factor (TNF) receptor superfamily, located on the surface of the cell fated for programmed death. Oligomerization of the ligand and Fas (or member of the TNF superfamily) occurs, which leads to the interaction of several key components including two death effector domain (DED)-containing proteins, the Fas-associated death domain (FADD), and the proenzyme form of caspase 8. When all this has occurred, procaspase 8 auto-activates and thus other downstream caspases cleave and are activated. Another way apoptosis can be induced is through hydrogen peroxide. Peroxides can be made by TNF, be produced by activated macrophages, neutrophils, or other inflammatory cells. Along with oxygen-reactive metabolites, hydrogen peroxide can cause purposeful cellular damage and sometimes apoptosis. Chemokines are a component of another apoptotic pathway. They are small signal proteins that bind to specific G-protein-coupled membrane receptors. By binding, chemokines can recruit leukocytes to the infected area [16].
Some of the better understood open reading frames that assist in molluscum contagiosum virus’ elusion of immune system response are MC159, MC160, MC66, and MC148. MC159 and MC160 have two tandem DEDs. MC159 binds FADD and procaspase-8, thus interfering with the cellular pathway to induce apoptosis. The mechanism of MC160 is not as well understood as that of MC159. It is suspected that it is involved in the inhibition of the Fas or TNF-induced apoptotic pathways [16]. The RxDL motif in the MC160 death effector domain-containing protein was previously shown to be required for MC160 protein function. In one study, it was hypothesized that in order for MC160 to function properly, the RxDL motif would need to inhibit activation of the host’s inflammatory response to the virus. They carried out this study by testing whether molluscum contagiosum viruses with mutated RxDL motifs could inhibit TBK1- and MAVS- induced activation of interferon beta. Interestingly, the researchers of this experiment found that the mutated virus was still able to stop the action of interferon beta. Thus, it is not the RxDL motif of the MC160 protein that is responsible for molluscum contagiosum virus’ interference with the host’s immune response [17]. Another open reading frame, MC66, contains a UGA codon and a hairpin structure, which are necessary genomic components in order to produce selenocysteine. When UGA is alternatively encoded, selenocysteine is incorporated. Selenocysteine breaks down hydrogen peroxide to water and oxygen and, in conjunction with superoxide dismutase and catalase, reduces the oxygen-reactive metabolites that can damage the cell or induce apoptosis. This dismantling of apoptosis-inducers allows GPx, a selenocysteine-containing protein, to avoid cell death and thus allows the molluscum contagiosum virus to continue replicating. MC148 codes for components that interfere with another pathway to apoptosis. Namely, it has been proposed that these components act as chemokine antagonists thus lymphocytes are unable to be activated, recruited, or differentiated. Other poxviruses, rather than code for a chemokine antagonist, encode for chemokine-binding proteins, which inhibit an inflammatory immune response [16].
More in-depth studies have delved into the exact methods of apoptotic elusion. For types I and II, the chemokine signal is antagonized and thus effector cells are not recruited to the site of infection. This finding explains the absence of T-cells and natural killer cells at the base of the molluscum regions; no signal is sent to summon them. It is also possible that as the lesion develops, a physical barrier is created which prevents the immune system from both detecting and resolving the infection. When lesions do resolve on their own, it is typically because one immune cell is eventually able to infiltrate. This emphasizes how important the immune response is to thwarting infection by molluscum contagiosum virus [18]. Another study further explored molluscum contagiosum virus’ ability to avoid immune response. Typically when an infection arises, NF-κB activation occurs due to pattern recognition receptor and cytokine signaling. So, in order for a virus to be detected, NF-κB must be activated. MC132 is a protein that is coded for by molluscum contagiosum virus and has been found to interact with p65, one of the NF-κB subunits. This interaction causes p65 to degrade. MC132 does this by ubiquitinating p65, making it a target for a complex in the host, Cullin-5/Elongin B/Enlongin C, which then degrades the subunit [19]. In this way, molluscum contagiosum virus uses a host’s immune system against itself.
Molluscum contagiosum virus and HIV
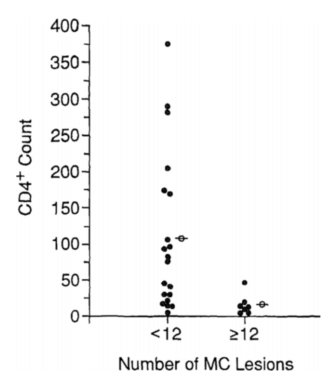
Molluscum contagiosum infection is opportunistic, meaning that it takes advantage of – and thus commonly infects – those with weakened immune systems, such as people with HIV/AIDs. It does so by taking advantage of their lowered CD4+ counts [20]. CD4 is a glycoprotein located on the surface of T helper cells, monocytes, and macrophages. Levels of CD4+ have been directly correlated with number and severity of molluscum contagiosum virus pustules. In one experiment, CD4+ levels were correlated with number of molluscum contagiosum lesions and it was found that patients with higher numbers (over 12) pustules had lower CD4+ counts than those with lower numbers (under 12) of pustles (Figure 4). Further, lower T4 lymphocyte counts have been correlated with higher incidence of cutaneous disorders [20]. Because it is opportunistic, molluscum contagiosum infection can be a sign of a weakening immune system [4], which is why it is also common in patients receiving chemotherapy or corticosteriods [20]. It has been found that between 5 to 18% of HIV infected person are also infected with molluscum contagiosum [20]. Not only are they more commonly infected, but immunocompromised individuals, such as those with HIV/AIDs can develop much more severe symptoms such as very large lesions (greater than 15 mm in diameter) and a greater number of lesions [10].
Typical therapies for immunocompetent patients are not as effective for immunocompromised patients, such as those with HIV/AIDs. It has been found that the most effective treatment for molluscum contagiosum infection are therapies that boost the immune system. Specifically for patients with HIV, systemic, antiviral, and immune-modulating drugs have been found to be more effective than any direct ablative methods [4]. For severe cases, pustules can be directly injected with interferon, a naturally occurring protein that is made by the immune system. This method has been used to treat facial lesions. However, this method is accompanied by undesirable side effects such as influenza-like symptoms, site tenderness, and lethargy [9].
References
- ↑ American Academy of Dermatology: Molluscum contagiosum. (2015). Retrieved from https://www.aad.org/public/diseases/contagious-skin-diseases/molluscum-contagiosum
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Birthistle, K., and Carrington, D. (1997). Molluscum contagiosum virus. Journal of Infection 34(1): 21-8.
- ↑ 3.0 3.1 MayoClinic. (2015). Molluscum contagiosum: Definition. Retrieved from http://www.mayoclinic.org/diseases-conditions/molluscum-contagiosum/basics/definition/con-20026391
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 Chen, X., Anstey, A.V., and Bugert, J.J. (2013). Molluscum contagiosum virus infection. The Lancet: Infectious Diseases 13(10): 877-88.
- ↑ 5.0 5.1 Centers for Disease Control and Prevention. (2015). Molluscum contagiosum. Retrieved from http://www.cdc.gov/poxvirus/molluscum-contagiosum/
- ↑ Sharquie, K.E., Hameed, A.F., and Abdulwahhab, W.S. (2015). Pathogenesis of molluscum contagiosum: a new concept for the spontaneous involution of the disease. Dermatology 3(3):265-9.
- ↑ Centers for Disease Control and Prevention. (2015). Transmission: molluscum contagiosum. Retrieved from http://www.cdc.gov/poxvirus/molluscum-contagiosum/transmission.html
- ↑ Centers for Disease Control and Prevention. (2015). Risk factors: molluscum contagiosum. Retrieved from http://www.cdc.gov/poxvirus/molluscum-contagiosum/risk.html
- ↑ 9.0 9.1 Centers for Disease Control and Prevention. (2015). Treatment options: molluscum contagiosum. Retrieved from http://www.cdc.gov/poxvirus/molluscum-contagiosum/treatment.html
- ↑ 10.0 10.1 10.2 Centers for Disease Control and Prevention. (2015). Clinical information: molluscum contagiosum. Retrieved from http://www.cdc.gov/poxvirus/molluscum-contagiosum/clinical_information.html
- ↑ Moye, V., Cathcart, S., Burkhart, C.N., and Morell, D.S. (2013). Beetle juice: a guide for the use of cantharidin in the treatment of molluscum contagiosum. Dermatologic Therapy 26(6): 445-51.
- ↑ Guan, H., Nuth, M., Zhukovskaya, N., Saw, Y.L., Bell, E., Isaacs, S.N., and Ricciardi, R.P. A novel target and approach for identifying antivirals against molluscum contagiosum virus. Antimicrobial Agents in Chemotherapy 58(12): 7383-9.
- ↑ Centers for Disease Control and Prevention. (2015). Prevention: molluscum contagiosum. Retrieved from http://www.cdc.gov/poxvirus/molluscum-contagiosum/prevention.html
- ↑ Senkevich, T.G., Koonin, E.V., Bugert, J.J., Darai, G., and Moss, B. "The genome of molluscum contagiosum virus: Analysis and comparison with other poxviruses." Virology. 1997. Volume 233(1). p. 19-42.
- ↑ Dohil, M.A., Lin, P., Lee, J., Lucky, A.W., Paller, A.S, and Eichenfield, L.F. (2006). The epidemiology of molluscum contagiosum in children. Journal of the American Academy of Dermatology 54(1): 47-54.
- ↑ 16.0 16.1 16.2 16.3 Moss, B., Shisler, J.L., Xiang, Y., Senkevich, T.G. (2000). Immune-defense molecules of molluscum contagiosum virus, a human poxvirus. Trends in Microbiology 8(10):473-7.
- ↑ Weber, S. (2015). Functional analysis of the molluscum contagiosum virus MC160 death effector domain-containing protein RxDL Motif. Seton Hall University Dissertations and Theses (ETDs). Paper 2068.
- ↑ Krathwohl, M.D., Hromas, R., Brown, D.R., Broxmeyer, H.E., and Fife, K.H. (1997). Functional characterization of the C–C chemokine-like molecules encoded by molluscum contagiosum virus types 1 and 2. PNAS 94(18):9875-80.
- ↑ Brady, G., Haas, D.A., Farrell, P.J., Pichlmair, A., and Bowie, A.G. (2015). Poxvirus protein MC132 from Molluscum contagiosum virus inhibits NF-kB activation by targeting p65 for degradation. Journal of Virology 89(16):8406-16.
- ↑ 20.0 20.1 20.2 20.3 20.4 Schwartz, J.J., and Myskowski, P.L. (1992). Molluscum contagiosum in patients with human immunodeficiency virus infection: A review of twenty-seven patients. Journal of the American Academy of Dermatology 27(4): 583-8.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
