Nematopsis
Classification
Higher order Taxa: Chromista (Kingdom) > Harosa (Subkingdom) > Alveolata (Infrakingdom) > Myzozoa (Phylum) > Apicomplexa (Subphylum) > Sporozoa (Infraphylum) > Conoidasida (Class) > Gregarinasina (Subclass) > Eugregarinorida (Order) > Septatorina (Suborder) > Porosporidae (Family) (1). Genus: Nematopsis
Description and significance
Nematopsis is a genus of eukaryotic, protozoic, gregarine parasites causing disease in both wild and cultured bivalves and crustaceans (2, 3, 4, 5, 6). Gregarines have been distinguished from Coccidia and Adeledis by ribosomal RNA gene sequences (7), and Nematopsis in particular has been distinguished from the closely related genus Porospora by its oocyst morphology (8). Nematopsis affects a wide array of commercially vital marine organisms by alternating hosts over the course of its life cycle (2, 3, 4, 6, 9, 10). Some countries that extensively harvest these species include Brazil, Thailand, China, the United States, and Spain, along with many other coastal nations (2, 3, 6, 7, 10). Nematopsis has received increasing attention since the aquaculture industry around the world has been affected by its presence. Observation of Nematopsis oocytes in marine organisms and some aspects of its life cycle have been well documented, but genomic and metabolic studies of this parasite are lacking, as well as the severity of its impact on wild and commercial marine ecosystems.
Genome structure
The ribosomal RNA of Nematopsis temporariae has been used to identify this species as an infectious agent in tadpoles (11). However, no other genetic material in the Nematopsis genus has been sequenced, so little is known about its particular genome structure. In lieu of genomic data, several factors are used to distinguish species within the genus Nematopsis, including: oocyte size (2), presence of microfibril structure in oocyst (2, 12), host specificity and distribution within the host (23). As of October 2016, there are 35 species within the Nematopsis genus that have been documented in the World Register of Marine Species (1).
Cell structure
Nematopsis oocytes are readily observed within the phagocytes of the infected tissues of host organisms (2). Thus, the morphology of Nematopsis oocytes is the most well-characterized out of their life cycle. Numerous oocytes can occupy a single phagocyte, each containing an oocyst wall and additionally protected within a parasitophorous (parasite-containing) vacuole (PV) in the cytoplasm (2). Some species have a mesh of microfibrils that projects from the outer oocyst wall to the PV (2, 12). The oocyte itself is apical, with its organelles concentrated at one pointed end that contains an opening called a micropyle covered by a canopy-like structure known as an operculum (2). Each oocyst contains a single uninucleated sporozoite, which distinguishes Nematopsis from the genus Porospora, which has multiple “naked” sporozoites within each oocyst (12). Nematopsis has a life cycle that is typical of its apicomplexian subphylum which includes formation of gametes (sex cells), development of protective spores, and multiplication through asexual reproduction (known as gametogony, sporogony, and schizogony, respectively) (8). The exact morphology of each stage varies by species (8).
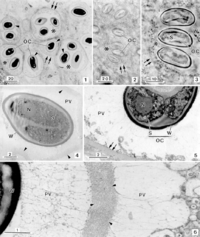
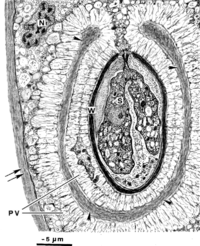
Metabolic processes
Due to the parasitic nature of Nematopsis, in vivo host tissues cultures have been underutilized in favor of in situ ecological studies. While there is a lack of research on the digestive metabolism within the entire Apicomplexan clade, it has been shown that the presence of Nematopsis alters the fat metabolism in host C. virginia oysters (13). This could suggest a defensive role on the host’s behalf to decrease uptake of essential fatty acids and lipids, or undescribed histopathological damage to host lipid metabolism. The genomic sequence of the closely related gregarine Ascogregarina taiwanensis (Apicomplexia) encodes enzymes including advanced cytochromes for oxidative electron transport chain, mitochondrial carrier proteins, relatively advanced mitochondrial organelles, and Krebs cycle proteins (14); this suggests that gregarines and likely also eugregarines (i.e., Nematopsis spp.) are capable of complete oxidative metabolism and exhibit high metabolic diversity within hosts. Considering the biassociative parasitic nature within the Nematopsis genus, the portions of the genome coding for digestive and intracellular metabolic pathways would logically express a plurality of protein carriers, synthases, and catalytic enzymes for host switching and planktonic stages. De novo amino acid synthases or amino acid synthases from intermediates for at least seven amino acids, as well as enzymes for de novo pyrimidine synthesis, have also been found in related organisms (14). Highly fecund parasitic Apicomplexa taxa (including Nematopsis) demand high rates of phospholipid biosynthesis to maintain infectious cells (15). Nematopsis have, consequently, developed unique secretory organelles called rhoptries for phospholipid and cholesterol storage; it has been suggested that rhoptries sequester phospholipids and cholesterol for rapid mitotic events and dispersal of newly split infectious cells.
Ecology
The degree of damage to hosts from Nematopsis parasitism is still being studied and quantified. One meta-analysis of commercially-exploited bivalves in “Ria de Vigo”, Galicia, NW Spain found that every individual of G. depressa, M. galloprovinciallis, S. vagina, and T. rhomboideus were actively parasitized by Nematopsis. This phenomenon raises interesting questions regarding shifting ecological states as a factor of Nematopsis parasitism as well as the integrity of bivalve biodiversity in heavily parasitized environments (5). Individual bivalves may be so heavily affected by gregarine parasitism that local lesions develop in the internal organs, particularly in the gills (9). How this localized damage can affect population integrity through inter- and intraspecific spreading is not known. Furthermore, the degree to which Nematopsis affects crustacean hosts is also understudied. The genus’ net annual economic damage to worldwide fisheries is unknown. Scattered studies have found Nemptopsis to be more prevalent in autumn (16), and in the morning and afternoon(17). These findings also open up questions of how changing ocean temperatures will affect the prevalence of Nematopsis.
Pathology
Although members of the Nematopsis genus have not been found to be human pathogens, they have nonetheless been found to infect various marine bivalves with commercial value (2, 3, 4, 6, 9, 10). It is unclear if Nematopsis is able to cause or assist in large-scale fatalities of its hosts (18). The host’s normal immunological response is ineffective against Nematopsis as the parasite lives within host phagocytes (12). Specific tissues targeted include: the hepatopancreas, abductor muscle, gills, mantle, and gonads. The exact tissues infected vary across different habitats, even in the same cultured oysters (4). Interestingly, gregarines in general exhibit gliding motility similar to that of plasmodium, which causes malaria, and toxoplasma, which causes taxoplasmosis, making these organisms potentially useful as a model in the study and treatment of these diseases (19, 20).
Current Research
As of 2016, there has been emphasis placed on the role of Nematopsis as parasites in commercially valuable marine species, particularly in coastal bivalves. The integrity of wild bivalve and crustacean stocks around the world is of economic and practical concern. Between 2012 and present-day (October 2016), almost all published literature on Nematopsis has focused on Nematopsis as a potential cause for the depleting health of shellfish stocks worldwide, with a select few papers about novel species within the genus and no literature on intracellular mechanics. Florencia et al. conducted a study in Chile on 57 bivalve species, many of which were commercially valuable, and sorted the individual sizes within the study based off of assumed market or harvest value (24). A similar study was lead with a focus on histopathological damage from Nematopsis on the edible Crassostrea madrasensis oyster and the implications on the wild oyster stocks (25). The health of five nearshore ‘commercially valuable’ Pakistani oyster species (Crassostrea, Saccostrea, and Ostrea) were collected and analyzed for parasites with particular attention to the presence of Nematopsis, which was generally found in individuals with other parasitic trematodes, perhaps shedding light on the opportunistic parasitic nature of Nematopsis (26).
References
[1] WoRMS (2015). Nematopsis. Accessed through: World Register of Marine Species at http://marinespecies.org/aphia.php?p=taxdetails&id=390581
[2] Azevedo, Carlos, and Isairas Padovan. "Nematopsis gigas n. sp.(Apicomplexa), a parasite of Nerita ascencionis (Gastropoda, Neritidae) from Brazil." Journal of Eukaryotic Microbiology 51.2 (2004): 214-219.
[3] Bower, S.M. (1996): Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish: Gregarine Disease of Penaeid Shrimp. Canadian Department of Fisheries and Oceans (September 1996). http://www.dfo-mpo.gc.ca/science/aah-saa/diseases-maladies/gregdpsp-eng.html
[4] Brito, Luis Otavio, José Carlos Nascimento, and Alfredo Olivera GáL Barros. "Presence of Nematopsis sp.(Protozoa, Apicomplexa) in the oyster, Crassostrea rhizophorae (Guilding, 1828), cultivated in the state of Pernambuco, Brazil." World aquaculture (2010). doi: 0.1007/s004360050655
[5] Soto, M., Pascual, S., Rodriguez, H., Gestal, C., Abollo, E., Arias, C., Estevez, J. Nematopsis Spp. Schneider, 1892 (Appicomplexa: Gregarinida) in Bivalve Molluscs Off Ria De Vigo (Galicia, NW Spain). (1996) Bulletin- European Association of Fish Pathologists, 16, 157-160.
[6] Tuntiwaranuruk, C., et al. "Investigation of Nematopsis spp. oocysts in 7 species of bivalves from Chonburi Province, Gulf of Thailand." Diseases of aquatic organisms 58.1 (2004): 47-53.
[7] Carreno R. A., Martin D. S., Barta J. R. (1999).Cryptosporidium is more closely related to the gregarines than to coccidia as shown by phylogenetic analysis of apicomplexan parasites inferred using small-subunit ribosomal RNA gene sequences. Parasitol. Res. 85 899–904. doi: 10.1007/s004360050655
[8] Prasadan, P. K.; Janardanan, K. P., 2001: Three new species of gregarines (Apicomplexa: Sporozoea: Porosporidae) in the estuarine crabs from Kerala, India. Acta Protozoologica 40(4): 303-309.
[9] Lauckner, G. "Diseases of mollusca: bivalvia." Diseases of marine animals 2 (1983).
[10] 2010 World Health Organization (WHO). Safe Management of Shellfish and Harvest Waters. Edited by G. Rees, K. Pond, D. Kay, J. Bartram and J. Santo Domingo. ISBN: 9781843392255. Published by IWA Publishing, London, UK.
[11] Chambouvet, A., Valigurová, A., Pinheiro, L. M., Richards, T. A. and Jirků, M. (2016), Nematopsis temporariae(Gregarinasina, Apicomplexa, Alveolata) is an intracellular infectious agent of tadpole livers. Environmental Microbiology Reports. doi:10.1111/1758-2229.12421
[12] Padovan,I., et al. “Fine structure of the oocyst of Nematopsis mytella (Apicomplexa, Porosporidae), a parasite of the mussel Mytella falcata and of the oyster Crassostrea rizophorae (mollusca, bivalvia) from the northeastern Atlantic coast of Brazil” Brazil J Morphology 20(3):141-145.
[13] Luz, Mariane dos Santos Aguiar, and Guisla Boehs. "Parasites in the oyster Crassostrea rhizophorae from farmed and natural stocks in the Bay of Camamu, Bahia, northeastern Brazil." Journal of Parasitology and Vector Biology 7.6 (2015): 120-128.
[14] Templeton, Thomas J., et al. "A genome-sequence survey for Ascogregarina taiwanensis supports evolutionary affiliation but metabolic diversity between a Gregarine and Cryptosporidium." Molecular biology and evolution 27.2 (2010): 235-248.
[15] Coppens, Isabelle, and Ole Vielemeyer. "Insights into unique physiological features of neutral lipids in Apicomplexa: from storage to potential mediation in parasite metabolic activities." International journal for parasitology 35.6 (2005): 597-615.
[16] Özer, A., & Güneydaʇ, S. (2015). Seasonality and host-parasite interrelationship of Mytilus galloprovincialis parasites in Turkish Black Sea coasts. Journal of the Marine Biological Association of the United Kingdom, 95(8), 1591–1599. doi: 10.1017/S0025315415000740
[17] Gutiérrez-Salazar, G. J., Molina-Garza, Z. J., Hernández-Acosta, M., García-Salas, J. A., Mercado-Hernández, R., & Galaviz-Silva, L. (2011). Pathogens in Pacific white shrimp (Litopenaeus vannamei Boone, 1931) and their relationship with physicochemical parameters in three different culture systems in Tamaulipas, Mexico. Aquaculture, 321(1–2), 34–40.
[18] Nascimento, Iracema Andrade, Donald H. Smith, Frederick Kern, and Solange Andrade Pereira. 1986. “Pathological Findings in Crassostrea Rhizophorae from Todos Os Santos Bay, Bahia, Brazil.” Journal of Invertebrate Pathology 47 (3): 340–49. doi:10.1016/0022-2011(86)90105-9.
[19] Ménard, R. (2001), Gliding motility and cell invasion by Apicomplexa: insights from the Plasmodium sporozoite. Cellular Microbiology, 3: 63–73. doi:10.1046/j.1462-5822.2001.00097.x
[20] Meissner, Role of Toxoplasma gondii Myosin A in Powering Parasite Gliding and Host Cell Invasion. Science 25 OCT 2002 : 837-840.
[21] USDA. Centers for Epidemiology & Animal Health. (1995). Overview of Aquaculture in the United States, 8117 (October).
[22] Cottee, S. Y., & Petersan, P. (2009). Animal Welfare and Organic Aquaculture in Open Systems. Journal of Agricultural and Environmental Ethics, 22(5), 437–461. doi:10.1007/s10806-009-9169-2
[23] Sprague, Victor, and P. E. Orr. 1955. “Nematopsis Ostrearum and N. Prytherchi (Eugregarinina: Porosporidae) with Special Reference to the Host-Parasite Relations.” The Journal of Parasitology 41 (1): 89. doi:10.2307/3274005.
[24] Cremonte, Florencia, et al. "Histopathological survey of the mussel Mytilus chilensis (Mytilidae) and the clam Gari solida (Psammobiidae) from southern Chile." (2015).
[25] Suja, G and Kripa, V and Mohamed, K S and Shamal, P and Sanil, N K (2016) Nematopsis sp. (Apicomplexa: Porosporidae) infection in Crassostrea madrasensis and its associated histopathology. Journal of the Marine Biological Association of India, 58 (1). pp. 29-33. ISSN 2321-7898
[26] Afsar, Nuzhat, Ghazala Siddiqui, and David Roberts. "Parasite Inspection in Five Commercially Important Oyster Species (Mollusca: Bivalvia) of Pakistan." Journal of Basic & Applied Sciences 10 (2014): 220.
Edited by students of Jennifer Talbot for BI 311 General Microbiology, 2016, Boston University.
