Nitrososphaera viennensis
A Microbial Biorealm page on the genus Nitrososphaera viennensis
Classification
Higher order taxa
Archaea; Thaumarchaeota; Nitrososphaerales; Nitrososphaeraceae
Species
Nitrososphaera viennensis
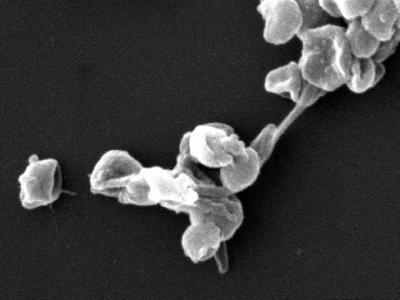
|
NCBI: Taxonomy |
Description and significance
N. viennensis is a slightly irregular, spherically shaped ammonia oxidizing archaeon with a cell diameter of approximately 0.5-0.8 μm(3). It was the first cultivated terrestrial archaeal ammonia oxidizer(3).
Etymology: L. adj. nitrosus, "full of natron," (nitrite producer); L. fem. n. sphaera, spherically shaped; L. viennensis, isolated from Vienna (3)
Genome structure
The sequence divergence of N. viennensis's 16s rRNA gene from its closest relative, Nitrososphaera gargensis, is 3%(3).
Ammonia oxidizing Bacteria aerobically oxidize ammonia using ammonia monooxygenase (amoA) to catalyze the initial oxidation of NH3(2). Ammonia oxidizing Archaea have amo-like genes that may contribute to their ammonia oxidation(2).
The urease gene cluster was located in one strain of N. viennensis, EN76(3). The cluster consisted of the encoding subunits ureA, ureB, and ureC and the accessory proteins ureE, ureF, ureG, and ureD(3).
|
NCBI: Genome |
Metabolism
N. viennensis used urea or ammonia as an energy source in laboratory conditions(3). It is able to grow chemolithoautotrohically with inorganic compounds as an electron donor, but displayed higher growth rates when grown with bacteria or with the addition of 0.1mM pyruvate(3). It is currently unclear whether it is capable of heterotrophic or mixotrophic growth.(3). The addition of vitamins, amino acids, and sugars had only a marginal effect on growth(3). Acetylene is a possible growth inhibitor(3).
Its optimal temperature is 35°C with an optimum pH of 7.5(3). Generation time of a pure culture with 1mM ammonia and pyruvate was about ~45h(3). Cell densities reached 5 X 107 cells mL-1(3). Growth on cultures with ammonium alone slowed down to ~23d demonstrating it can grow autotrophically exclusively although very slowly(3).
It is able to withstand higher ammonia concentrations than other ammonia oxidizing archaea such as Nitrosopumilus maritimus and Nitrososphaera gargensis (3). Growth occurred at concentrations as high as 15 mM, but was inhibited at 20 mM(3). Inhibitory ammonia concentrations for N. maritimus and N. gargensis are 2 to 3 mM. Ammonia tolerances were similar to some oligotrophic bacterial ammonia oxidizers associated with the Nitrosomonas oligotropha cluster(3). However, other bacterial ammonia oxidizers have a much higher maximum ammonia tolerance of 50 to 1,000 mM(3).
The electron transfer process is not fully understood yet in ammonia oxidizing archaea(2). The amount of ATP per mole of NH3 is unknown since it varies depending on growth stage and other factors(2). Lack of cytochrome c proteins and many copper-containing proteins suggest a different electron transport mechanism than ammonia oxidizing bacteria, which is iron-heme-dependent(2).
Ecology
N. viennensis exists in soil globally(2). The first N. viennensis strain isolated was from garden soil from the University Center at Althanstrasse in Vienna's 9th District(4).
Ammonia oxidizing Archaea such as N. viennensis may contribute to the nitrification of the environment(3). It was previously believed only Bacteria could oxidize ammonia(3). Ammonia oxidation is the first step in denitrification (NH3 to NO2-) (2). Eventually N2O is produced in this process and escapes into the environment (2). N2O has a greenhouse warming potential 310 times higher than CO2(2). It is responsible for 5%-7% of the greenhouse effect(2). It is the third most important greenhouse gas after CO2 and CH4(2). Ammonia oxidizing Bacteria and Archaea are estimated to contribute to ~70% of global N2O emissions(2).
Evidence shows that ammonia oxidizing Bacteria and Arachaea work together in mangrove forests in nitrogen cycling(1). These highly productive forests are important natural habitats for many marine organisms(1). Coastal ecosystems benefit from nutrient transformation and transport provided by ammonia oxidizing Bacteria and Archaea(1). Natural and man-made mangrove wetlands have been used as low cost way to treat municipal sewage with high amounts of inorganic nitrogen, which can be transformed by ammonia oxidizing Bacteria and Archaea(1).
References
1. Li, M., Cao, H., Hong, Y., and J. Gu (2011) Spatial distribution and abundances of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) in mangrove sediments, Environmental Biotechnology 89: 1243-1254; doi: 10.1007/s00253-010-2929-0
2. Hatzenpichler, R. (2012) Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea, Applied and Environmental Microbiology 2012 Nov: 78(21): 7501-10; doi: 10.1128/AEM.01960-12
3. Tourna, M., Stieglmeier, M., Spang, A., Könneke, M., Schintlmeister, A., Urich, T., Engel, M., Schloter, M., Wagner, M., Richter, A., and C. Schleper (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil, PNAS 2011; doi:10.1073/pnas.1013488108
4. University of Vienna (2011) Novel microorganism 'Nitrososphaera viennensis' isolated. ScienceDaily, 2011 April 28. Retrieved April 28, 2013, from http://www.sciencedaily.com/releases/2011/04/110425153556.htm
Edited by Shanna Lovelace of Dr. Lisa R. Moore, University of Southern Maine, Department of Biological Sciences, http://www.usm.maine.edu/bio
