Oral Microbiome and Cognitive Decline
Introduction to the Oral Microbiome
By Evan Dean '23
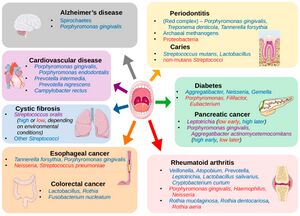
The oral microbiome is a dynamic and intricate ecosystem of microorganisms inhabiting the oral cavity, playing a pivotal role in maintaining oral and overall health [1]. It comprises a diverse array of bacteria, fungi, viruses, and protozoa, with more than 700 bacterial species and 1,000 phylotypes identified to date [2] [3]. Similar to fingerprints, the oral microbiome exhibits substantial variability, making it a unique feature of each person [4].
The oral microbiome's composition and function are influenced by various factors, including genetics, diet, oral hygiene, and environmental exposures [3]. Bacterial species in the oral cavity can be broadly categorized into three groups: commensals, opportunistic pathogens, and pathogens [1]. Commensal bacteria are generally harmless and can even confer health benefits, while opportunistic pathogens may cause disease under certain conditions. In contrast, pathogens are consistently associated with disease states [2]. Recent research has emphasized the importance of the oral microbiome in human health, revealing its connection to various systemic conditions such as cardiovascular diseases, diabetes, and even cognitive function [5] [2]. (Figure 1) Among the hundreds of bacterial species in the oral microbiome, some well-known representatives include Streptococcus mutans, associated with dental caries, and Porphyromonas gingivalis, linked to periodontal disease [3]. Other notable bacteria include the nitrogen-fixing Fusobacterium nucleatum, which plays a critical role in the development of dental plaque, and the highly diverse Actinomyces species, involved in the formation of dental biofilms [2]. The balance between pathogenic and commensal bacteria is essential for maintaining oral health, and disruptions in this equilibrium can lead to oral infections and other complications [1]. For instance, dysbiosis, an imbalance in the microbial community, can result in the overgrowth of pathogenic species, contributing to periodontitis and other oral diseases [5]. Advances in molecular techniques, such as oligotyping, have allowed scientists to explore the human oral microbiome at a higher resolution, uncovering its complex structure and interactions [4]. This deeper understanding has led to the development of novel strategies for promoting oral health, as well as the prevention and treatment of oral diseases [2]. The relationship between the oral microbiome and cognitive function is an emerging area of research. While a direct link is yet to be firmly established, there is evidence suggesting that chronic oral infections, such as periodontitis, can contribute to systemic inflammation and the release of pro-inflammatory cytokines, which may in turn affect cognitive function [5]. Some studies have identified specific oral pathogens, such as Porphyromonas gingivalis, in the brains of Alzheimer's disease patients, implying a potential connection between the oral microbiome and neurodegenerative disorders [2]. These findings highlight the need for further investigation to better understand the interplay between oral health, the microbiome, and overall well-being.
Alzheimer's Disease Overview
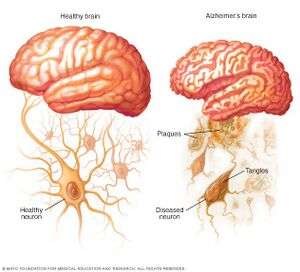
Alzheimer's disease (AD) is the most common form of dementia, affecting millions of people worldwide and posing significant public health challenges [6]. It is a progressive neurodegenerative disorder characterized by cognitive decline, memory loss, and impaired daily functioning [7]. The exact cause of AD remains unclear, but it is believed to involve a complex interplay of genetic, environmental, and lifestyle factors [8].
At the cellular level, two hallmark features of AD are the presence of extracellular amyloid plaques and intracellular neurofibrillary tangles [7]. Amyloid plaques are primarily composed of aggregated amyloid-β (Aβ) peptides, which result from the cleavage of amyloid precursor protein (APP) by β- and γ-secretases [8]. The accumulation of Aβ peptides is thought to play a critical role in the pathogenesis of AD, although the exact mechanisms by which they contribute to neurodegeneration remain under investigation [6].
Increasing evidence suggests that bacteria may play a role in the development of AD. One hypothesis is that certain bacterial infections, particularly those involving the oral cavity, can induce systemic inflammation and the production of pro-inflammatory cytokines, which may contribute to neuroinflammation and the accumulation of amyloid plaques [8]. In fact, some studies have identified specific oral pathogens, such as Porphyromonas gingivalis, in the brains of AD patients, supporting the potential link between oral microbiome dysbiosis and AD [7].
Given the significant public health burden of AD, understanding the potential role of bacteria and other factors in its pathogenesis is critical for developing effective prevention and treatment strategies [6].
Amyloid Plaque and Neurofibrillary Tangles
Amyloid plaques and neurofibrillary tangles are two hallmark pathological features of Alzheimer's disease. Both amyloid plaques and neurofibrillary tangles have been associated with synaptic dysfunction, neuronal loss, and impaired brain function [9] [10]. Amyloid plaques are extracellular deposits composed primarily of β-amyloid (Aβ) peptides, which are derived from the proteolytic cleavage of the amyloid precursor protein (APP). The aggregation of Aβ peptides into insoluble fibrils leads to the formation of amyloid plaques [11]. These plaques can disrupt synaptic communication, leading to neuronal dysfunction and ultimately cell death [12]. In addition, activated microglia and astrocytes, which are glial cells involved in the brain's immune response, have been shown to surround amyloid plaques, contributing to the inflammatory response in Alzheimer's disease [13]. Neurofibrillary tangles, on the other hand, are intracellular aggregates of hyperphosphorylated tau protein. Tau is a microtubule-associated protein that helps maintain the stability of microtubules in neuronal axons. In Alzheimer's disease, abnormal hyperphosphorylation of tau leads to the formation of paired helical filaments, which aggregate into neurofibrillary tangles [10]. These tangles disrupt the neuronal cytoskeleton, impairing axonal transport and ultimately leading to neuronal death [9]. While amyloid plaques and neurofibrillary tangles are characteristic of Alzheimer's disease, they can also be found in the brains of cognitively normal older adults, suggesting a complex relationship with the natural aging process [10]. It is important to note that the presence of these pathological features does not necessarily lead to clinical symptoms, as other factors, such as synaptic resilience and compensatory mechanisms, can influence cognitive function in the aging brain [12]. Recent evidence has suggested that oral bacteria, particularly those associated with periodontal disease, can contribute to the development of amyloid plaques and neurofibrillary tangles in Alzheimer's disease. For instance, Porphyromonas gingivalis, a keystone pathogen in periodontitis, has been detected in the brains of Alzheimer's patients, and its toxic proteases (gingipains) have been found to co-localize with tau and Aβ [14]. This suggests a possible link between oral bacteria and the pathological features of Alzheimer's disease. Oral bacteria, particularly those related to periodontal disease, may contribute to the development of these pathological features, emphasizing the importance of oral health in maintaining cognitive function throughout aging.
Inflammatory Mediators
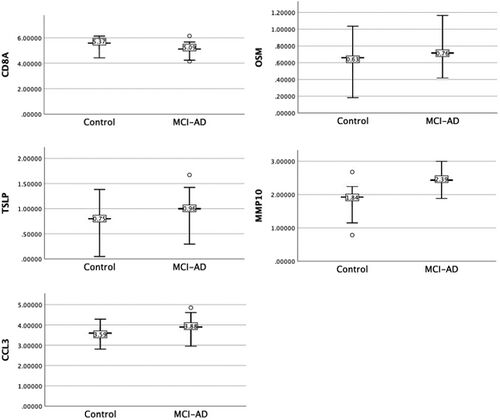
The connection between oral bacteria and Alzheimer's disease (AD) has been increasingly explored in recent years, with a particular focus on the role of inflammatory mediators in this relationship. Oral bacteria, particularly periodontal pathogens, can stimulate the host immune system, leading to the production of pro-inflammatory cytokines. Some of the main periodontal pathogens implicated in this process are Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola [15]. These pathogens have been found to trigger the immune system, resulting in the release of cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) (Pritchard et al. 2017). The pro-inflammatory cytokines produced in response to oral bacteria can lead to systemic inflammation, which is thought to contribute to the pathogenesis of AD [16]. Systemic inflammation has been linked to several key features of AD pathology, including the accumulation of amyloid-β (Aβ) peptides and the formation of neurofibrillary tangles (NFTs) [17]. Yang et al. (2021) investigated the oral microbiome and inflammation in individuals with mild cognitive impairment (MCI), a precursor to AD. The study found that MCI participants had an altered oral microbiome composition compared to cognitively normal individuals, as shown in (Figure 3). Additionally, MCI participants exhibited higher levels of pro-inflammatory cytokines, such as IL-6 and TNF-α. These findings suggest that the oral microbiome may be involved in the inflammatory processes contributing to the development of AD. Further research has indicated that the presence of periodontal pathogens in the bloodstream may lead to the production of inflammatory mediators, which can cross the blood-brain barrier (BBB) and enter the central nervous system (CNS) [18]. Once inside the CNS, these inflammatory mediators may contribute to neuroinflammation. [19].
Narengaowa et al. (2021) [19] proposed that the oral-gut-brain axis could be a potential mechanism by which oral bacteria contribute to AD. In this proposed model, oral bacteria could reach the gut and impact the gut microbiome composition. The gut microbiome, in turn, could contribute to the production of inflammatory mediators, which would affect the CNS and potentially lead to the development of AD. The production of pro-inflammatory cytokines by oral bacteria is a key factor connecting the oral microbiome with the development and progression of AD. The underlying mechanisms may involve systemic inflammation, the crossing of inflammatory mediators through the BBB, and the influence of the oral-gut-brain axis. Further research is needed to fully understand the role of inflammatory mediators in this relationship and to explore potential therapeutic approaches targeting oral bacteria and inflammation in AD prevention and treatment.
Proteases
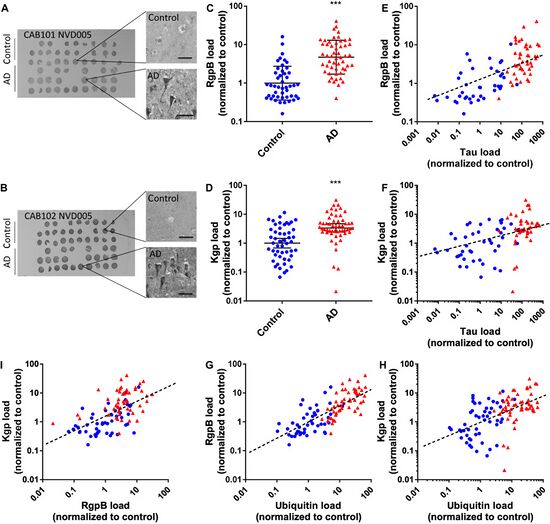
The potential connection between oral bacteria and Alzheimer's disease (AD) is not limited to the production of inflammatory mediators. Recent research has also focused on specific proteases produced by oral bacteria and their possible involvement in the development and progression of AD. In this section, we will discuss the role of these proteases and the underlying mechanisms that may link them to AD. Porphyromonas gingivalis, a key periodontal pathogen, produces a group of proteases known as gingipains [14]. Gingipains, which include lysine-specific gingipain (Kgp) and arginine-specific gingipains (RgpA and RgpB), are thought to play a critical role in the virulence and pathogenicity of P. gingivalis [14]. Importantly, Dominy et al. (2019)[14] found that gingipains were present in the brains of AD patients, suggesting a potential link between P. gingivalis and AD pathology. Figure 4 demonstrates the presence of P. gingivalis-derived gingipains. The presence of gingipains in the AD brain may contribute to neuroinflammation and neuronal damage through multiple mechanisms. One such mechanism involves the cleavage of host proteins by gingipains, which could lead to the production of toxic protein fragments that promote inflammation and neuronal injury [20]. Additionally, gingipains may contribute to the formation of amyloid-β (Aβ) plaques, a hallmark of AD pathology, by cleaving amyloid precursor protein (APP) in a manner that promotes Aβ production [20]. Other oral bacteria, such as Treponema denticola and Tannerella forsythia, also produce proteases that could potentially contribute to AD pathology [21]. These proteases might have similar effects on the host's proteins, promoting inflammation and neurotoxicity [21]. However, further research is needed to fully understand the role of proteases from different oral bacteria in AD development and progression. Research by Panza et al. (2019)[22] suggests that targeting oral bacteria and their proteases could be a promising therapeutic approach for AD. In their review, the authors discuss the potential benefits of antibacterial therapies in preventing or treating AD, with a focus on the role of proteases. They argue that inhibiting bacterial proteases, such as gingipains, could help reduce the neuroinflammatory response and limit the production of toxic protein fragments, thereby potentially mitigating AD progression. In conclusion, proteases produced by oral bacteria, such as gingipains from P. gingivalis, may play a significant role in the development and progression of AD. These proteases could contribute to neuroinflammation and neuronal damage by cleaving host proteins and promoting the formation of toxic protein fragments and Aβ plaques. Further research is needed to better understand the precise mechanisms by which oral bacterial proteases contribute to AD pathology and to explore potential therapeutic strategies targeting these proteases for AD prevention and treatment.
Periodontal Disease
The oral microbiome is a complex ecosystem of microorganisms that inhabit the oral cavity, playing a crucial role in maintaining oral health [23]. Imbalances in the oral microbiome can lead to various oral diseases, including periodontal disease, which is characterized by inflammation and destruction of the supporting structures of the teeth [24]. Periodontal disease is a multifactorial disease involving interactions between the oral microbiome and the host's immune system [25]. Specific bacterial strains have been implicated in the initiation and progression of periodontal disease, such as Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola [25]. These pathogenic bacteria form biofilms on tooth surfaces, which can lead to the destruction of periodontal tissues and alveolar bone loss [23]. The progression of periodontal disease can result in tooth mobility, tooth migration, and ultimately tooth loss [26]. As a consequence of tooth loss, occlusal imbalance can occur, which refers to the disruption of the normal contact and force distribution between the upper and lower teeth during mastication [26]. Occlusal imbalance may lead to further oral health issues, such as temporomandibular joint disorders, increased tooth wear, and changes in the overall structure and function of the remaining dentition [26]. Periodontal disease not only affects the oral microbiome and local oral health but also has broader implications for overall health. The presence of periodontal pathogens, such as P. gingivalis, can lead to systemic inflammation and contribute to the pathogenesis of other chronic inflammatory diseases, such as cardiovascular disease, diabetes, and even Alzheimer's disease [27]. The oral microbiome is intimately connected to periodontal health, and alterations in the oral microbiome can lead to the development of periodontal disease. Periodontal disease, in turn, can result in tooth loss and occlusal imbalance, further impacting oral health and overall well-being. Maintaining a balanced oral microbiome through proper oral hygiene and regular dental checkups is essential to prevent periodontal disease and its consequences on occlusal balance and systemic health.
Occlusal Imbalance
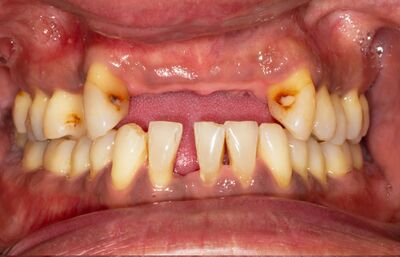
Occlusal imbalance, characterized by an improper alignment of the teeth, has been suggested to contribute to cognitive decline and Alzheimer's disease through multiple mechanisms. A poor oral microbiome can lead to periodontal disease, which in turn may result in tooth loss, malocclusion, and eventually, occlusal imbalance. There is a wide range of recent research that looks at the relationship between occlusal imbalance and cognitive decline. The research looks at the various potential mechanisms that connect the two. Regarding the potential consequences of occlusal imbalance, De Cicco et al. (2016)[28] found that occlusal disharmony could negatively impact nerve function, leading to reduced cortical responsiveness in the somatosensory cortex of rats. Furthermore, Tramonti Fantozzi et al. (2019) [29] reported that occlusal disharmony led to changes in the expression of genes related to neuroplasticity in the rat hippocampus, indicating a potential mechanism through which occlusal imbalance could contribute to cognitive decline. Tooth loss, which can result from periodontal disease (Figure 5), has also been implicated in cognitive decline through multiple mechanisms. Goto et al. (2020) [30] found that losing molars in rats led to reduced cerebral blood flow in the somatosensory cortex, resulting in neuronal death and potentially impairing cognitive function. Luo et al. (2019) [31] also reported that rats with molar loss exhibited decreased spatial cognitive performance, which was associated with reduced hippocampal neurogenesis. Takeda et al. (2016) [32] demonstrated that tooth loss and a powdered diet could alter mRNA expression of brain-derived neurotrophic factor (BDNF) in the hippocampus of adult mice. BDNF is a gene which is a critical component in neuronal survival, synaptic plasticity, and cognitive function [33] [34]. Another interesting connection is that tooth loss has been associated with brain atrophy [35], apoptosis [36], and alterations in hormone regulation [37]. These can contribute to cognitive decline and the development of Alzheimer's disease. Overall, a poor oral microbiome and subsequent periodontal disease may lead to occlusal imbalance and tooth loss, which can have detrimental effects on cognitive function through various mechanisms, including inflammation, neuroplasticity, cerebral blood flow, and gene expression. Maintaining good oral health and addressing occlusal imbalance may, therefore, play a crucial role in preventing cognitive decline and the development of Alzheimer's disease.
Conclusion
This page highlights the intricate relationship between oral health, particularly the oral microbiome, and the development of Alzheimer's disease. The oral cavity is home to a diverse range of microorganisms, including bacteria that play a crucial role in maintaining overall oral health. An imbalance in the oral microbiome, particularly the presence of pathogens associated with periodontal disease, can contribute to systemic inflammation, which has been implicated in the pathogenesis of Alzheimer's disease. The presence of oral bacteria, such as Porphyromonas gingivalis, has been linked to the formation of hallmark Alzheimer's pathological features, including amyloid plaques and neurofibrillary tangles. A thorough understanding of the mechanisms through which oral bacteria and their byproducts contribute to Alzheimer's disease could lead to novel preventive and therapeutic strategies. Research investigating the role of oral microbiome dysbiosis in Alzheimer's pathogenesis is still in its early stages, and there is much to be explored. Future work could focus on elucidating the molecular pathways involved in the interaction between oral bacteria and Alzheimer's pathology, as well as on identifying potential biomarkers for early detection and intervention. The development of novel therapeutics targeting specific oral pathogens or their toxic byproducts could help mitigate Alzheimer's disease progression. Longitudinal studies examining the impact of oral health interventions on cognitive function and Alzheimer's risk are also warranted to determine the potential benefits of improved oral hygiene and periodontal care. Ultimately, the recognition of oral health as an important factor in the development and progression of Alzheimer's disease underscores the need for a multidisciplinary approach to prevention and treatment. By integrating dental care into the broader healthcare landscape, and by fostering collaboration between dental and medical professionals, we can work towards improving the quality of life for individuals at risk of or suffering from Alzheimer's disease, and potentially uncover new avenues for the prevention and treatment of this neurodegenerative disorder.
References
- ↑ 1.0 1.1 1.2 [Wade, William G. “The Oral Microbiome in Health and Disease.” Pharmacological Research, SI:Human microbiome and health, 69, no. 1 (March 1, 2013): 137–43. https://doi.org/10.1016/j.phrs.2012.11.006. ]
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 [Verma, Digvijay, Pankaj Kumar Garg, and Ashok Kumar Dubey. “Insights into the Human Oral Microbiome.” Archives of Microbiology 200, no. 4 (May 1, 2018): 525–40. https://doi.org/10.1007/s00203-018-1505-3.]
- ↑ 3.0 3.1 3.2 [Deo, Priya Nimish, and Revati Deshmukh. “Oral Microbiome: Unveiling the Fundamentals.” Journal of Oral and Maxillofacial Pathology : JOMFP 23, no. 1 (2019): 122–28. https://doi.org/10.4103/jomfp.JOMFP_304_18. ]
- ↑ 4.0 4.1 [Deo, Priya Nimish, and Revati Deshmukh. “Oral Microbiome: Unveiling the Fundamentals.” Journal of Oral and Maxillofacial Pathology : JOMFP 23, no. 1 (2019): 122–28. https://doi.org/10.4103/jomfp.JOMFP_304_18.]
- ↑ 5.0 5.1 5.2 [Jenkinson, Howard F. “Beyond the Oral Microbiome.” Environmental Microbiology 13, no. 12 (2011): 3077–87. https://doi.org/10.1111/j.1462-2920.2011.02573.x.]
- ↑ 6.0 6.1 6.2 [Ballard, Clive, Serge Gauthier, Anne Corbett, Carol Brayne, Dag Aarsland, and Emma Jones. “Alzheimer’s Disease.” The Lancet 377, no. 9770 (March 19, 2011): 1019–31. https://doi.org/10.1016/S0140-6736(10)61349-9.]
- ↑ 7.0 7.1 7.2 [Scheltens, Philip, et al. "Alzheimer's disease." The Lancet 388.10043 (2016): 505-517 https://doi.org/10.1016/S0140-6736(15)01124-1]
- ↑ 8.0 8.1 8.2 [Querfurth, Henry W., and Frank M. LaFerla. “Alzheimer’s Disease.” New England Journal of Medicine 362, no. 4 (January 28, 2010): 329–44. https://doi.org/10.1056/NEJMra0909142.]
- ↑ 9.0 9.1 [Gómez-Isla, Teresa, Richard Hollister, Howard West, Stina Mui, John H. Growdon, Ronald C. Petersen, Joseph E. Parisi, and Bradley T. Hyman. “Neuronal Loss Correlates with but Exceeds Neurofibrillary Tangles in Alzheimer’s Disease.” Annals of Neurology 41, no. 1 (1997): 17–24. https://doi.org/10.1002/ana.410410106. ]
- ↑ 10.0 10.1 10.2 [Guillozet, Angela L., Sandra Weintraub, Deborah C. Mash, and M. Marsel Mesulam. “Neurofibrillary Tangles, Amyloid, and Memory in Aging and Mild Cognitive Impairment.” Archives of Neurology 60, no. 5 (May 1, 2003): 729–36. https://doi.org/10.1001/archneur.60.5.729.]
- ↑ [Gouras, Gunnar K., Tomas T. Olsson, and Oskar Hansson. “β-Amyloid Peptides and Amyloid Plaques in Alzheimer’s Disease.” Neurotherapeutics 12, no. 1 (January 1, 2015): 3–11. https://doi.org/10.1007/s13311-014-0313-y.]
- ↑ 12.0 12.1 [Mathis, C. A., Y. Wang, and W. E. Klunk. “Imaging β-Amyloid Plaques and Neurofibrillary Tangles in the Aging Human Brain.” Current Pharmaceutical Design 10, no. 13 (May 1, 2004): 1469–92. https://doi.org/10.2174/1381612043384772.]
- ↑ [Nagele, Robert G, Jerzy Wegiel, Venkat Venkataraman, Humi Imaki, Kuo-Chiang Wang, and Jarek Wegiel. “Contribution of Glial Cells to the Development of Amyloid Plaques in Alzheimer’s Disease.” Neurobiology of Aging, Challenging Views of Alzheimer’s Disease - Round II, 25, no. 5 (May 1, 2004): 663–74. https://doi.org/10.1016/j.neurobiolaging.2004.01.007.]
- ↑ 14.0 14.1 14.2 14.3 [Dominy, Stephen S., Casey Lynch, Florian Ermini, Malgorzata Benedyk, Agata Marczyk, Andrei Konradi, Mai Nguyen, et al. “Porphyromonas Gingivalis in Alzheimer’s Disease Brains: Evidence for Disease Causation and Treatment with Small-Molecule Inhibitors.” Science Advances 5, no. 1 (January 23, 2019): eaau3333. https://doi.org/10.1126/sciadv.aau3333.]
- ↑ [Pritchard, Anna B., StJohn Crean, Ingar Olsen, and Sim K. Singhrao. “Periodontitis, Microbiomes and Their Role in Alzheimer’s Disease.” Frontiers in Aging Neuroscience 9 (2017). https://www.frontiersin.org/articles/10.3389/fnagi.2017.00336.]
- ↑ [Olsen, Ingar, and Sim K. Singhrao. “Can Oral Infection Be a Risk Factor for Alzheimer’s Disease?” Journal of Oral Microbiology 7, no. 1 (January 1, 2015): 29143. https://doi.org/10.3402/jom.v7.29143. ]
- ↑ [Yang, Irene, Robert Adam Arthur, Liping Zhao, Jasmine Clark, Yijuan Hu, Elizabeth J. Corwin, and James Lah. “The Oral Microbiome and Inflammation in Mild Cognitive Impairment.” Experimental Gerontology 147 (May 1, 2021): 111273. https://doi.org/10.1016/j.exger.2021.111273. ]
- ↑ [Sureda, Antoni, Maria Daglia, Sandro Argüelles Castilla, Nima Sanadgol, Seyed Fazel Nabavi, Haroon Khan, Tarun Belwal, et al. “Oral Microbiota and Alzheimer’s Disease: Do All Roads Lead to Rome?” Pharmacological Research 151 (January 1, 2020): 104582. https://doi.org/10.1016/j.phrs.2019.104582.]
- ↑ 19.0 19.1 [Narengaowa, Wei Kong, Fei Lan, Umer Farooq Awan, Hong Qing, and Junjun Ni. “The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease.” Frontiers in Cellular Neuroscience 15 (2021). https://www.frontiersin.org/articles/10.3389/fncel.2021.633735. ]
- ↑ 20.0 20.1 [Block, Janice. “Alzheimer’s Disease Might Depend on Enabling Pathogens Which Do Not Necessarily Cross the Blood-Brain Barrier.” Medical Hypotheses 125 (April 1, 2019): 129–36. https://doi.org/10.1016/j.mehy.2019.02.044. ]
- ↑ 21.0 21.1 [Dibello, Vittorio, Madia Lozupone, Daniele Manfredini, Antonio Dibello, Roberta Zupo, Rodolfo Sardone, Antonio Daniele, Frank Lobbezoo, and Francesco Panza. “Oral Frailty and Neurodegeneration in Alzheimer’s Disease.” Neural Regeneration Research 16, no. 11 (March 25, 2021): 2149–53. https://doi.org/10.4103/1673-5374.310672.]
- ↑ [Panza, Francesco, Madia Lozupone, Vincenzo Solfrizzi, Mark Watling, and Bruno P Imbimbo. “Time to Test Antibacterial Therapy in Alzheimer’s Disease.” Brain 142, no. 10 (October 1, 2019): 2905–29. https://doi.org/10.1093/brain/awz244. ]
- ↑ 23.0 23.1 [Costalonga, Massimo, and Mark C. Herzberg. “The Oral Microbiome and the Immunobiology of Periodontal Disease and Caries.” Immunology Letters, Microbiome influences on host immunity, 162, no. 2, Part A (December 1, 2014): 22–38. https://doi.org/10.1016/j.imlet.2014.08.017.]
- ↑ [Pihlstrom, Bruce L, Bryan S Michalowicz, and Newell W Johnson. “Periodontal Diseases.” The Lancet 366, no. 9499 (November 19, 2005): 1809–20. https://doi.org/10.1016/S0140-6736(05)67728-8. ]
- ↑ 25.0 25.1 [Di Stefano, Mattia, Alessandro Polizzi, Simona Santonocito, Alessandra Romano, Teresa Lombardi, and Gaetano Isola. “Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment.” International Journal of Molecular Sciences 23, no. 9 (January 2022): 5142. https://doi.org/10.3390/ijms23095142.]
- ↑ 26.0 26.1 26.2 [Passanezi, Euloir, and Adriana Campos Passanezi Sant’Ana. “Role of Occlusion in Periodontal Disease.” Periodontology 2000 79, no. 1 (2019): 129–50. https://doi.org/10.1111/prd.12251.]
- ↑ [Niemiec, Brook A. “Periodontal Disease.” Topics in Companion Animal Medicine, Veterinary Dentistry, 23, no. 2 (May 1, 2008): 72–80. https://doi.org/10.1053/j.tcam.2008.02.003. ]
- ↑ [De Cicco, Vincenzo, Massimo Barresi, Maria Paola Tramonti Fantozzi, Enrico Cataldo, Vincenzo Parisi, and Diego Manzoni. “Oral Implant-Prostheses: New Teeth for a Brighter Brain.” PLoS ONE 11, no. 2 (February 26, 2016): e0148715. https://doi.org/10.1371/journal.pone.0148715.]
- ↑ [Tramonti Fantozzi, Maria Paola, Stefano Diciotti, Carlo Tessa, Barbara Castagna, Daniele Chiesa, Massimo Barresi, Giulio Ravenna, et al. “Unbalanced Occlusion Modifies the Pattern of Brain Activity During Execution of a Finger to Thumb Motor Task.” Frontiers in Neuroscience 13 (May 17, 2019): 499. https://doi.org/10.3389/fnins.2019.00499.]
- ↑ [Goto, Takaharu, Nobuaki Higaki, Takahiro Kishimoto, Yoritoki Tomotake, and Tetsuo Ichikawa. “Does Periodontal Tactile Input Uniquely Increase Cerebral Blood Flow in the Prefrontal Cortex?” Brain Sciences 10, no. 8 (August 2020): 482. https://doi.org/10.3390/brainsci10080482.]
- ↑ [Luo, Bin, Qian Pang, and Qingsong Jiang. “Tooth Loss Causes Spatial Cognitive Impairment in Rats through Decreased Cerebral Blood Flow and Increased Glutamate.” Archives of Oral Biology 102 (June 1, 2019): 225–30. https://doi.org/10.1016/j.archoralbio.2019.05.004.]
- ↑ [Takeda, Yosuke, Hiroshi Oue, Shinsuke Okada, Akira Kawano, Katsunori Koretake, Makoto Michikawa, Yasumasa Akagawa, and Kazuhiro Tsuga. “Molar Loss and Powder Diet Leads to Memory Deficit and Modifies the MRNA Expression of Brain-Derived Neurotrophic Factor in the Hippocampus of Adult Mice.” BMC Neuroscience 17, no. 1 (December 5, 2016): 81. https://doi.org/10.1186/s12868-016-0319-y.]
- ↑ [Erickson, Kirk I., Ruchika Shaurya Prakash, Michelle W. Voss, Laura Chaddock, Susie Heo, Molly McLaren, Brandt D. Pence, et al. “Brain-Derived Neurotrophic Factor Is Associated with Age-Related Decline in Hippocampal Volume.” The Journal of Neuroscience 30, no. 15 (April 14, 2010): 5368–75. https://doi.org/10.1523/JNEUROSCI.6251-09.2010.]
- ↑ [Dou, Shu-Hui, Yu Cui, Shu-Ming Huang, and Bo Zhang. “The Role of Brain-Derived Neurotrophic Factor Signaling in Central Nervous System Disease Pathogenesis.” Frontiers in Human Neuroscience 16 (2022). https://www.frontiersin.org/articles/10.3389/fnhum.2022.924155. This demonstrates the possibility of changes in gene expression that can result from poor oral health. ]
- ↑ [Kobayashi, Takuya, Masafumi Kubota, Toshiyuki Takahashi, Ayaka Nakasato, Taro Nomura, Junichi Furuya, and Hisatomo Kondo. “Effects of Tooth Loss on Brain Structure: A Voxel-Based Morphometry Study.” Journal of Prosthodontic Research 62, no. 3 (July 2018): 337–41. https://doi.org/10.1016/j.jpor.2017.12.007.]
- ↑ [Mori, Daisuke, Hidekazu Miyake, Kenmei Mizutani, Kan Shimpo, Shigeru Sonoda, Toshiharu Yamamoto, Shuu Fujiwara, and Kin-ya Kubo. “Effects of Occlusal Disharmony on the Hippocampal Dentate Gyrus in Aged Senescence-Accelerated Mouse Prone 8 (SAMP8).” Archives of Oral Biology 65 (May 1, 2016): 95–101. https://doi.org/10.1016/j.archoralbio.2016.01.015.]
- ↑ [Furukawa, Masae, Hirobumi Tada, Jingshu Wang, Mitsuyoshi Yamada, Mie Kurosawa, Akiko Satoh, Noboru Ogiso, Yosuke Shikama, and Kenji Matsushita. “Molar Loss Induces Hypothalamic and Hippocampal Astrogliosis in Aged Mice.” Scientific Reports 12 (April 18, 2022): 6409. https://doi.org/10.1038/s41598-022-10321-w.]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2023, Kenyon College
