Parvimonas micra
Classification
Higher order taxa
Bacteria – Firmicutes – Tissierellia – Tissierellales – Peptoniphilaceae – Parvimonas [1]
Species
Parvimonas micra (P. micra) and type of strain includes ATCC 33270, CCUG 46357, CIP 105294, DSM 20468, GIFU 7824, JCM 12970, NCTC 11808, and VPI 5464 [2].
Description and significance
P. micra is a gram positive cocci that forms pairs and groups of short chains, where it is not associated with the formation of spores [3, 4]. The bacterium is an obligate anaerobe and was first identified and classified in 1933 as Peptostreptococcus micros [3, 5]. The species was then reclassified in 1999 as Micromonas micros and again in 2006 as Parvimonas micra [5]. Since its discovery, the bacterium has been deemed a commensal pathogen in the human oral cavity, where it is most abundant in the subgingival dental plaque [3, 4, 6].
P. micra can be observed via culturing, where anaerobic culture vials and blood agar can be used to achieve observable colonies [3]. Incubation period is suggested to be around 48 hours [3]. The importance of this bacterium regarding research would be its implication in pathogen mediated diseases in humans, such as periodontitis [7].
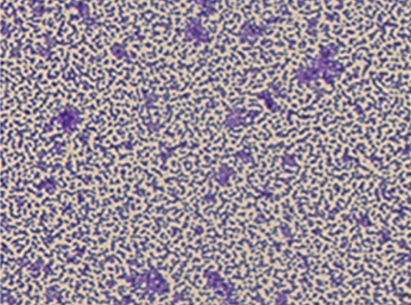
Figure 1: Purple staining indicates the presence of P. micra, where the stain colouration is associated with gram positive bacteria [16].
Genome structure
Genome structural analysis of P. micra based on strain A293 displayed a genome size of 1,680,794bp, where it had a G + C content of 28.3% [4]. The strain contained 49 contigs, where contig size varied from 539bp to 407,487bp [4]. Further genome analysis displayed that the genome comprises of 1,560 coding sequences and 46 RNAs, where 40 represents tRNAs and 6 rRNAs [4].
The total number of genes detected in the A293 strain was around 430 genes [4]. From this, 137 genes are associated with the metabolism of proteins, 60 are involved the processing carbohydrates, 86 are essential in amino acid synthesis, 58 are key in DNA synthesis, and 89 are related to RNA synthesis [4]. The 62 genes out of the 89 genes associated with RNA synthesis were identified to be involved in nucleotide formation and nucleoside synthesis, while 3 genes out of 89 genes are related with prophages, phage, plasmids, and transposable elements [4].
Cell structure and metabolism
P. micra is a gram-positive bacterium, which indicates that a large composition of its cell wall is made up of a thick layer of peptidoglycan [3]. P. micra has no flagella and is associated with biofilms in the subgingival dental plaque of the human oral cavity [7]. Often observed as micro-colonies in the top layer of these biofilms, its presence has pathogenic potential and can give rise to periodontitis [7]. Scientific finding has suggested that P. micra resides in biofilms that have already been developed, as it has no ability to form structures associated with biofilm formation [8].
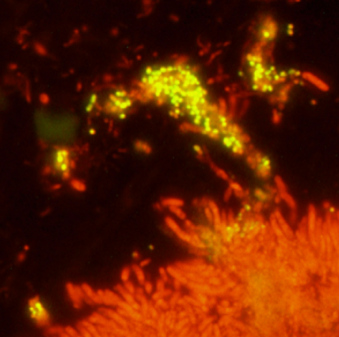
Figure 2: Cluster of P. micra bacterial cells indicated using a yellow fluorescence, where they are detected in the top layer of the biofilm associated with the sub-gingival dental plaque [7].
Ecology
P. micra is an obligate anaerobe that resides in the human oral cavity, where it is most abundant in the subgingival dental plaque as a commensal pathogen [3, 4, 6]. In addition, P. micra is also identified to inhabit the gastrointestinal tract [9]. Furthermore, it is suggested that P. micra has a potential to be a constitute of the vaginal flora; however, further research is required for verification [3, 10].
Pathology
P. micra is regarded as one of the pathogens that causes periodontitis in humans, which is a commonly observed disease [7, 8]. The pathogenesis of periodontitis involves the damage of tissue structures that support the teeth, such as the gums, the alveolar bone, and periodontal ligament, due to bacterial infection and inflammation [11, 12]. In addition, the disease is associated with severe complications if treatment is not provided early and adequately, as there is potential for it to spread to surrounding tissues [8].
Regarding pathogenesis, P. micra, is associated with the disturbance of the composition and abundance of commensal microorganisms established within biofilms that coats the subgingival dental plaque [8]. Therefore, a disturbance that leads to a slight increase in the abundance of pathogenic bacterial species, especially P. micra, would increase the likelihood of an infection or a disease to occur, such as periodontitis.
P. micra has also been documented to be involved in polymicrobial infections with Aggregatibacter aphrophilus, where it causes pleural empyema [13]. In addition, P. micra has been recently documented to cause endocarditis, where the bacterium was identified through molecular sequencing [14]. Furthermore, P. micra has been recently identified to be associated with septic arthritis of native joints, which suggests that this bacterium could be a potential pathogen for infections associated with joints and bones [9].
Other infections and diseases associated with P. micra includes peritonsillar abscesses, respiratory infections, pleural empyema, intracranial abscesses, paranasal sinus infections, spondylodiscitis, pericarditis, and septic embolism [3, 14, 15]. The ability of P. micra to cause infection in other sites of the human body apart from the oral cavity is suggested to be due to the translocation of the bacterium from the oral cavity or from the gastrointestinal tract [14]. However, further research is required to investigate the mechanisms behind this process as well as examining the genes and structures within this bacterium that are involved as virulence factors.
Application to biotechnology
Currently, there is limited use of P. micra in biotechnology, which includes the development of antibiotics and antibacterial monomers for use in periodontitis and cavity disinfection [16, 17]. This is due to P. micra having the potential to mediate infection and disease, such as periodontitis [14].
Current research
Recent findings regarding P. micra includes recently documented diseases that the bacterium can potentially cause, such as endocarditis [12]. Another recent discovery include how P. micra can be involved in polymicrobial infections with Aggregatibacter aphrophilus, where it causes pleural empyema [13]. Furthermore, it has been documented that P. micra could be a potential pathogen associated with joint and bone infections, where a recent case study has discovered that septic arthritis of native joints can be caused by this bacterium [9].
A recent literature has displayed interaction that occurs in the subgingival dental plaque between a bacterium of candidate division TM7 and other bacterial species, such as P. micra [18]. Certain bacterial species studied by the literature are associated with the potential to mediate periodontitis [18]. The interaction identified included morphological change that occured in TM7 bacteria, where it changed from a cocci to a rod, when it was in close proximity with P. micra bacteria [18]. This change is due to the variations in metabolic conditions and signalling within the biofilm, where further research could provide understanding about these bacterial species and their potential to mediate disease in a biofilm environment [18].
References
1. Taxonomy – Parvimonas micra
16. [http://www.joponline.org/doi/abs/10.1902/jop.2011.100657 Mouratidou, A., D’Hoedt, B., Karbach, J., Al-Nawas, B. (2011) Antibiotic Susceptibility of Cocultures in Polymicrobial Infections Such as Peri-Implantitis or Periodontitis: An In Vitro Model. Journal of Periodontology 82: 1360-1366.]
This page is written by Tristan Villamor for the MICR3004 course, Semester 2, 2017