Phytophthora infestans
Classification
Domain: Eukaryota; Phylum: Heterokontophyta; Class: Oomycota; Order: Peronosporales; Family: Pythiaceae; Genus: Phytopthora
Species
Phytophthora infestans
|
NCBI: [3] |
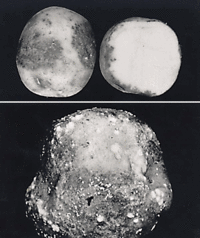
Description and Significance
Phytophtora infestans is an oomycete protist. P. infestans was originally thought to be a fungal species due to its filamentous structure and metabolic strategies, but recent biochemical and phylogenetic analyses has revealed that P. infestans is only remotely related to fungi and is more closely related to heterokont algae (12, 16). P. infestans growth structure is fungal-like, with the mycelium usually coenocytic and consisting of hyphae 5 to 8 µm in diameter (6). P. insfestans cell walls are primarily composed of cellulose and β-glucans and lack chitin (3). P. infestans is the causal agent of late blight in potatoes, lead to the Great Irish Famine in the mid nineteenth century, and causes approximately US $3 billion dollars worth damage annually world-wide (4). P. infestans is distributed worldwide, including major potato growing regions in Europe, North America, and South America (6).
Genome Structure
In 2009 the Phytophthora infestans genome was sequenced (7). The genome size is estimated to be 237 Mb consisting of a high G-C content, about 52% (15, 13). Microscopic analysis showed that there are 8-10 chromosomes (13). It is a diploid organism, meaning there are two sets of the chromosomes present within each cell (2).
Cell Structure, Metabolism and Life Cycle
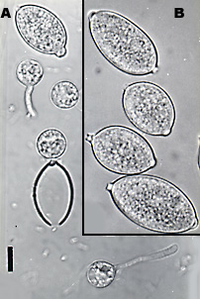
Life Cycle
Phytophthora have two kinds of resting or survival spores that they produce, both of which have thick cell walls that allow them to survive in suboptimal environments for long time periods. The two kinds of spores are oospores, formed from sexual recombination and chlamydospores, asexual survival spores(8).
Some species of Phytophthora are homothallic and some are heterothallic. Homothallic species can reproduce with just one thallus being present. Heterothallic species can reproduce only if two different thalli interact from mating types A1 and A2 (14). Phytophthora infestans is heterothallic(6).
Under cool wet conditions, Phytophthora oospores or chlamydospores which will germinate to form hyphae or directly produce sporangia. The sporangia are able to detach and travel in the air(6). The sporangia then release free swimming zoospores that have two flagella, one tinsel and one flagellate. The flagella allow the zoospore to travel in water on the surface of leaves as well as in the soil (6). Once a zoospore reaches the plants root or leaf surface it forms a cyst. The encysted zoospore then germinates and forms hyphae on the host surface. The tube like hyphae invades the plant leaf or root and allows the cell to get food (8).
After the Phytophthora infects the plant, it can produce sporangia and zoospores to further infect other tissues of the same plant or nearby plants. The Phytophthora will then produce more survival structures, oospores or chlamydospores, to overwinter and provide a source of inoculum for the following year’s life cycle(9).
Metabolism
Once the plant host has been infected the hyphae are used to take up nutrients. Nutrients required for growth are organic carbon source, oxygen, phosphorus, nitrogen, hydrogen, sulfur, calcium, magnesium, and iron(10).
Cell Structure:
The cell wall is composed mostly of (1-3) β-D-glucans, (1-6) β-D-glucans, and cellulous(3).
Ecology and Pathogenesis
As previously discussed, P. infestans is an economically significant plant pathogen and is most infamous because of the Great Irish Famine of the mid nineteenth century. The pathogen may be found in foliar regions of host plants and in soil. P. infestans inoculum survival and pathogenicity in soils may be attributed to a number of factors including soil texture, moisture content, and pH. P. infestans sporangia survival and pathogenicity is maximized in soils with increasing amounts of clay or loam, high amounts of moisture (20% moisture capacity), and generally decreases as soils become more acidic (ph of 3.8-4.2). (11, 1).
P. infestans is susceptible to inhibition and destruction by other microorganisms found in soils, including bacteria and fungi (11, 1). Mycelial growth of P. infestans occurs readily in sterilized soil but is strongly inhibited in unsterilized soil, which may be due to microorganisms populating the soil (17).
According to Erwin and Ribeiro (6), late blight epidemics caused by P. infestans are preceded by the following environmental coniditions: “1) night temperatures below the dew point for at least 4 hours; 2) low night temperature that does not drop below 10C; 3) the mean period of cloudiness not less than 0.8 of a day; and 4) rainfall of at least 0.1 mm on the following day.”
References
1)Andrivon, D. (1994), “Dynamics of the survival and infectivity to potato tubers of sporangia of Phytophthora infestans in three different soils.” Soil Biology Biochemistry, 26, 945-952.
2) Brasier, C. M. 1992. Evolutionary biology of Phytophthora. Part I: Genetic system, sexuality and the generation of variation. Annu. Rev. Phytopathol. 30:153-171.
3) Bartnicki-Garcia, S. (1968). Cell wall chemistry, morphogenesis and taxonomy of fungi. Annu. Rev. Microbiol. 22 87–108. 4)Duncan, J. (1999), “Phytophthora- an abiding threat to our crops.” Microbiology Today, 26, 114-116.
6) Erwin, Donanld and Ronald Olaf. Phytophthora Diseases Worldwide. St. Paul: The American Phytopathological Society, 1996. (6)Goodwin, S.B., Cohen, B.A., and Fry, W.E. (1994), “Panglobal distribution of a single clonal lineage of the Irish potato famine fungus.” Proceedings of the National Academy of Sciences USA, 91, 11591-11595.
7) Haas BJ, Kamoun S, Zody MC, Jiang RH, Handsaker RE, Cano LM, Grabherr M, Kodira CD, Raffaele S, Torto-Alalibo T, Bozkurt TO, Ah-Fong AM, Alvarado L, Anderson VL, Armstrong MR, Avrova A, Baxter L, Beynon J, Boevink PC, Bollmann SR, Bos JI, Bulone V, Cai G, Cakir C, Carrington JC, Chawner M, Conti L, Costanzo S, Ewan R, Fahlgren N, et al.: “Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans.” Nature 2009, 461:393-398.
8) Hardham, A. R. 2001. The cell biology behind Phytophthora pathogenicity. Australas. Plant Pathol. 30: 91-98 9) Hirst, J. M. and Stedman, O. J. 1960. The epidemiology of Phytophthora infestans. II. The source of inoculums. Ann. Appl. Biol. 48:489-517 10) Hohl, H.R. 1991. Nutrition, p. 53-83. In D.S. Ingram and P.H. Williams, (ed), Advances in Plant Pathology. Academic Press, Inc., San Diego, CA. 11)Lacey, J. (1965), “The infectivity of soils containing Phytophthora infestans.” Annals of applied Biology, 56, 363-380.
12)Kumar, S., and Rzhetsky, A. (1996), “Evolutionary relationships of Eukaryotic Kingdoms.” Journal of Molecular Evolution, 42, 183-193.
13)Sansome, E. and Brasier, C.M. (1973). Diploidy and chromosomal structural hybridity in Phytophthora infestans. Nature 241:344-345.
14) Savage, E. J., Clayton, C. W., Hunter, J. H., Brenneman, J. A., Laviola, C., Gallegly, M. E. 1968. Homothallism, heterothallism and interspecific hybridization in the genus phytophthora. Phytopathology 58:1004-1021.
15)Tooley, P.W. and Therrien, C.D. (1987). Cytophotometric determination of the nuclear DNA content of 23 Mexican and 18 non-Mexican isolates of Phytophthora infestans. Exp. Mycol. 11:19-26. (16)Van de Peer, Y., and De Watcher, R. (1997), “Evolutionary relationships among the eukaryotic crown taxa taking into account site-to-site variation in 18s rRNA.” Journal of Molecular Evolution, 45, 619-630.
(17)Zan, K. (1962), “Activity of Phytopthora infestans in soil in relation to tuber infection.” Transactions of the British Mycological Study, 45, 205-221.
Author
Page authored by Jayme Olsen and Pete Nelson, students of Prof. Jay Lennon at Michigan State University.
<-- Do not remove this line-->
