Platypus Evolution
Introduction
Platypuses (Ornithorhynchus anatinus) are monotremes, a type of egg-laying mammal with mammary glands and a flat beak. Aboriginal people in different regions have different names for the animal, including mallingong, boondaburra, and tambreet.[1] Platypuses are the only remaining member of the family Ornithorhynchidaeare, and there are only four other species of egg-laying mammals. Clearly, platypuses are unique. They live in the permanent river systems of eastern Australia, Tasmania, King’s Island, and Kangaroo Island, distributed across various environments ranging from tropical to alpine. Despite their range of environments, however, the IUCN deemed them “Near Threatened” in 2016.[2]
Platypuses are sexually dimorphic with the males approximately 15% longer and 40% heavier than the females. These differences suggest that males compete with each other for access to females, resources, and territory. They range from 700g to 3kg in mass. They are seasonal breeders and start earlier in the year farther north. Courtship (in which a couple dances on water) begins in August, and mothers start making elaborate nesting burrows for their eggs. Their young emerge anytime from late January to early March. Most platypuses live for six to fifteen years, but they can live into their twenties. They have small eyes with round pupils and flattened corneas, features of aquatic vertebrates. They are crepuscular/nocturnal and burrow to avoid high temperatures. Platypuses are aquatic feeders that close their eyes, nostrils, and ears while they forage underwater. They utilize an electro-sensory system in their bill to locate their prey (mostly invertebrates).[3] However, their eyes are sensitive to movement when open. [4]

Genetics
Fossil Record
The earliest known ancestors of modern platypuses lived during the early Cretaceous Period around 100 to 146 million years ago. Judging from fossil evidence, these monotremes are not very similar to modern platypuses, but a lower jaw fragment of Steropodon galmani from over 100 million years ago has platypus-like features and suggests that the mammal may have possessed a bill. The oldest known animal that is definitely a member of the platypus family is Monotrematum sudamericanum, which lived in Patagonia (South America) about 61 to 63 million years ago. At that time, Australia and South America were connected, forming a continent with Antarctica known as Gondwana.[1]
Researchers have also uncovered a fragment of a Pliocene platypus, Ornithorhynchus agilis, from roughly 3.8 million years ago. Another species, Obdurodon insignis, was identified based on two teeth in southern Australia that date back to the Oligocene approximately 26 Mya. More fossils have since been found. Fossils of Obdurodon dicksoni were in freshwater limestone from the Middle Miocene, around 15 Mya, and a larger species, Obdurodon tharalkooschild, was also found in the same area.[4]
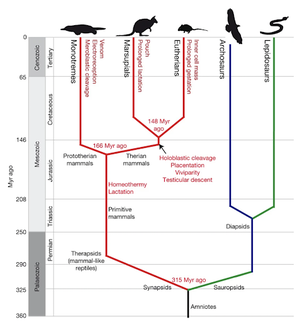
Phylogeny
Platypuses are monotremes, one of the three major mammalian lineages. The order has two extant families, the Ornithorhynchidae, which are semi-aquatic, and the Tachyglossidae, which are terrestrial. Platypuses are Ornithorhynchids. Monotremes (prototherian mammals) diverged from therians around 187 mya (Figure 2 indicates that the divergence occurred 166 mya, but that phylogenetic map, despite being the most informational one available, was created in 2008. There is no consensus, but the estimate of 187 mya may be more reliable as the research that yielded it is current, from a 2021 paper). The two monotreme groups diverged around 55 mya (split not shown in Figure 2). This estimate for the monotreme–therian split is earlier than previous estimates, which centered around 21 mya. The estimate of 55 million years is supported by recent analyses of fossil evidence and a few genes assuming that the gene substation rate is the same for all mammals.[2]
Echidnas are the closest living relatives of platypuses, and they belong to the family Tachyglossidae. They have very different appearances but share key features. Both groups have a single hole for all waste excretion and reproduction, the feature that inspired the name "monotreme." Both groups lack teeth because they lost four of the eight genes needed for tooth development. This suggests that echidnas and platypuses share a common toothless ancestor. They also both lay eggs and have relatively low active body temperatures for mammals. Platypuses and short-beaked echidnas also possess furless bills/beaks with receptors that detect the electrical fields of their prey. Adult males of both groups have pointed spurs on their ankles. The spurs of platypuses are connected to functional poison glands, but those of echidnas are not.[1] The common ancestor likely had spurs, and platypuses independently evolved venom, which contains at least 19 substances, thanks to duplications in β-defensin, C-type natriuretic peptide, and nerve growth factor gene families. Interestingly, reptiles gained their venom due to the same duplications, a case of convergent evolution.[3]]] The skeletons of echidnas and platypuses both have many reptilian features that are not present in other mammals, adding to the similarity between platypuses and reptiles. The earliest known echidna fossils are 15 million years old at most, so little is known about the evolutionary relationship between the two groups. However, researchers currently believe that echidnas developed from a platypus-like ancestor between 18 and 90 Mya.[1]
The platypus genome contains many traces of its reptilian ancestors. Male and female platypus karyotypes have 52 chromosomes; most are small with a few large ones, similar to the macro- and microchromosomes of reptiles. Platypuses have multiple sex chromosomes that are somewhat homologous to the Z chromosome of birds. Males have five X and five Y chromosomes that make a chain during meiosis and segregate into 5X and 5Y sperm.[3]
Unique Features/Adaptations
William Caldwell sent a telegram to the British Association that said, "Monotremes oviparous, ovum meroblastic," to announce that monotremes are different from marsupials and eutherians. Platypuses lay eggs, and eggs only divide partially. Other mammals such as humans and kangaroos give birth to live young and are holoblastic. Female platypuses do not possess nipples, so for 4 months, babies suck milk out of their mother's abdominal skin as most of their organ systems differentiate. The protein composition changes during lactation it does in marsupials but not in most eutherians. Platypuses have filiform sperm cells like birds and reptiles do, but they are the only amniote species that make bundles of 100 sperm cells when they through the epididymis. As in therians, platypus chromosomes are arranged in a certain order in their sperm. Their testes also synthesize testosterone and dihydrotestosterone, but there they do not have scrotums and their testes are in their abdomen.[3]
Their eyes contain double cones, which are not present in marsupials or eutherians. Their cochleas are also less sensitive than those of other mammals. The trigeminal nerve relays messages from specialized sensory structures in the pores on their bills and frontal shield. Rows of these electroreceptors run lengthwise with uniformly distributed mechanoreceptors. Thanks to these sensors, platypus bills provide electric and tactile information, helping the platypus navigate and find the weak electric fields made by their macroinvertebrate prey. They store food in cheek pouches.[4]
To avoid hypoxia when diving, platypuses have high hematocrit and erythrocyte counts, high hemoglobin levels, a low mean corpuscular volume, and a high mean corpuscular hemoglobin concentration. Their heart rates decrease when they are underwater, and they stay there for about a minute while foraging. Platypuses swim with their large, webbed front limbs pushing alternate strokes. They can swim at speeds around 0.7–3.6 km/h. They usually forage for 8–16 hours per day but can forage for more than 30 hours at a time. Unfortunately, they are not as good at walking. Their body is not always above ground when they haul themselves across the ground with splayed limbs. They expend 19–27% more energy when walking than most terrestrial mammals of a similar size.[4]
The tail has little fur and is mostly used for fat storage. It contains about 40% of the platypus' total body fat, though body fat percentage fluctuates with the seasons. Metabolism rises in the winter and during the breeding season.[4]
The platypus' genome protects it well as it has at least 214 natural killer receptor genes in the natural killer complex, an enormous number in comparison to humans, which have 15 genes, rats, which have 45, and opossums, which only have 9. The platypus genome also has gene expansions in the cathelicidin antimicrobial peptide gene family. Many eutherians do not have extra cathelicidin genes, but the gene expansions in marsupials and monotremes may give their young a variety of innate immune responses. An expansion in the macrophage differentiation antigen CD163 gene family is also present in the platypus genome.[3]
Their venom glands are a distinctive trait because there are few extant mammals with poison. The glands are on the surface of the pelvis, connected to spurs on both back legs by ducts. Female juveniles lose their vestigial spur sheaths by the time they turn one, but males keep both functional spurs. The male venom glands may have evolved from sweat glands. They enlarge during the breeding season, corresponding with increases in venom production and aggression. The venomous spurs may have evolved as a defense mechanism as hypothesized for Mesozoic mammals, but they are mostly for intraspecies competition now as platypuses do not have many native predators. The venom itself is chemically complex, created by 88 toxin genes. It disrupts cell membranes, hemostasis, and nociception (pain) but is not fatal with one spurring. The production of venom is cyclical, supporting the idea that platypus venom is for reproductive competition. It may cause temporary partial paralysis in other male platypuses and agonizing local pain in humans. On the bright side, platypus venom may lead to clinically useful substances and improve our understanding and treatment of pain.[4]
Microbes
Platypus milk contains an antimicrobial protein unique to monotremes — monotreme lactation protein (MLP). MLP likely evolved to eliminate microbial infections since the lack of nipples increases the risk.[4] The milk also contains a variegated array of at least twenty neutral and acidic oligosaccharides. All identified acidic oligosaccharides in platypus milk but 3′-sialyl lactose contains the 4-O-acetyl-sialic acid moiety, a unique feature shared with echidnas. The 4-O-acetylation of sialic acid moieties is what protects acidic milk oligosaccharides secreted onto integumental surfaces from hydrolysis by bacteria via steric interference with bacterial sialidases. Ancestral taxa of monotremes and other mammals likely secreted milk or a milk-like fluid with oligosaccharides onto skin surfaces, so this protection against bacteria may be evolutionarily significant.[5]
Many infectious agents have been isolated from platypuses but few cause serious disease. Mucormycosis caused by Mucor amphibiorum, an environmental fungus first detected in platypuses in 1982, causes significant mortality. It causes a lethal infection in Tasmanian platypuses and poses a considerable threat to mainland platypus populations if introduced.[6]. Mucormycosis causes severe granulomatous and often ulcerative dermatitis that sometimes reaches the underlying tissues or disseminates to the lungs. The effects of the pathogen are illustrated in Figure 3. Secondary bacterial infections or impaired thermoregulation and mobility can also lead to death. Corynebacterium ulcerans/non-Mucor fungal skin disease can cause similar infections and cutaneous foreign body reactions.[4]

Furthermore, researchers have found that a putative papilloma virus causes webbing papules and a virus similar to adenovirus causes a cytomegalic inclusion renal disease. In Victoria, 25% of necropsied platypus histologically suggested nephritis. In New South Wales, between 47% and 66% of platypuses had leptospirosis based on serology. Another threat is the Ixodes ornithorhynchi tick, which may cause mild dermatitis and be a vector of the hemoparasites Theileria ornithorhynchi and Trypanosoma binneyi. The former can cause hemolytic anemia in immunocompromised platypuses.[4] So, while platypuses have evolved strong immune systems, many microbes still pose a threat.
Conclusion
Overall, platypuses are unique among mammals. They resemble ducks, rodents, and a variety of other creatures, but their special path along the evolutionary tree distinguishes them and draws the curiosity of evolutionary biologists looking to map the relationships between fellow mammals. Their strange characteristics range from poisonous spurs to electroreceptors on a flat-billed beak, but all these traits are just adaptations to their environment that developed over millions of generations. But even with abilities that sound fictional and gene expansions boosting their innate immune responses, platypuses are not immune to danger. Mucormycosis has claimed the lives of countless platypuses in Tasmania, a reminder that all species are susceptible to pathogens.
References
- ↑ 1.0 1.1 1.2 1.3 “Evolution & Names.” Australian Platypus Conservancy, 4 Oct. 2021, https://platypus.asn.au/evolution-names/#:~:text=Aboriginal%20people%20had%20many%20different,lonely%20and%20persuasive%20water%2Drat.
- ↑ 2.0 2.1 Zhou, Yang, et al. "Platypus and echidna genomes reveal mammalian biology and evolution." Nature 592.7856 (2021): 756-762.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 [https://www.nature.com/articles/nature06936 Warren, Wesley C., et al. “Genome Analysis of the Platypus Reveals Unique Signatures of Evolution.” Nature, vol. 453, no. 7192, 8 May 2008, pp. 175–183., https://doi.org/10.1038/nature06936.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 https://academic.oup.com/jmammal/article/100/2/308/5477503# Bino, Gilad, et al. "The platypus: evolutionary history, biology, and an uncertain future." Journal of mammalogy 100.2 (2019): 308-327.
- ↑ Urashima, Tadasu, et al. "4-O-Acetyl-sialic acid (Neu4, 5Ac2) in acidic milk oligosaccharides of the platypus (Ornithorhynchus anatinus) and its evolutionary significance." Glycobiology 25.6 (2015): 683-69
- ↑ Connolly, Joanne H. "A review of mucormycosis in the platypus (Ornithorhynchus anatinus)." Australian Journal of Zoology 57.4 (2009): 235-244.
Edited by Cindy Chen, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2022, Kenyon College.
