Salmonella enterica serovar Typhi

Etiology/Bacteriology
Taxonomy
| Domain = Bacteria
| Phylum = Proteobacteria
| Class = Gammaproteobacteria
| Order = Enterobacteriales
| Family = Enterobacteriaceae
| Genus = Salmonella
| species = S. enterica
| serotype = Typhi
Description
Salmonella enterica serovar Typhi is a gram-negative, rod-shaped facultative anaerobe that only infects humans. It is unclear to scientists as to why this pathogen does not infect other organisms and has such a selective host behavior. When this bacteria first enters the human body it initially propagates inside the intestinal tract and spreads throughout the peripheral lymphatic system, such as the bone marrow and Peyer’s patches, to cause typhoid fever [3]. This disease has an incubation period of 1 to 2 weeks before the onset of any initial symptoms. This period may vary depending on the host’s immune system and the severity of the infection. It generally occurs in four stages throughout a four-week period [4]. Beginning with a fever between 103-104°F, the patient’s condition will continue to deteriorate due to symptoms including diarrhea, rashes, and delirium. Occasionally, complications from illnesses such as myocarditis, intestinal bleeding, or perforations will result, which often lead to patient death [5].
This pathogen has thrived in environments with unsanitary condition, especially in crowded areas, for thousands of years. The word Typhi was originally derived from the ancient Greek work typhos, or an ethereal smoke or cloud believed to cause madness and disease. Some scientists and historians have suggested that this bacterium may have been responsible for the Plague of Athens in the final stages of the Peloponnesian War. Antibiotics have reduced the frequency of typhoid fever, yet it still remains prevalent in developing countries. The relatively new serotype, S. Paratyphi causes similar, albeit milder symptoms with a more abrupt onset and a shorter course of infection. Both S. Typhi and S. Paratyphi serovars are referred to collectively as typhoidal Salmonella [6].
Typhoid fever is most commonly treated with antibiotics such as Ciprofloxacin, a second-generation fluoroquinolone. Due to the overuse of this medication throughout the past 10 years, antibiotic resistant strains have been discovered. For example, the clonal expansion of the haplotype H58 has increased in Asia and Africa where the invasive Salmonella Typhi continues to constitute a severe risk. However, other strains and haplotypes of typhoid fever remain sensitive to antibiotics despite the growing selection for antibiotic resistant strains [7]. Additional experimentation and research regarding Salmonella Typhi is difficult due to its preference for human hosts. Mouse models infected with S. enterica serovar Typhimurium have been the main source to observe pathogenic effects due to its similar genome, which differ only by 11% [8] [9].
Pathogenesis
Transmission
The transmission of Salmonella enterica serovar Typhi, like most Samonella serovars, occurs through the fecal-oral route. The pathogen generally spreads through contaminated food and water sources. Some hosts of Salmonella Typhi become asymptomatic carriers and can unknowingly transmit the pathogen to others. Because they are unaware of their condition, these carriers often do not take proper preventative measures for slowing the spread of bacteria. Without proper prevention, the bacteria are able to attain a high transmission rate. While lacking symptoms themselves, these carriers excrete large amounts of the bacteria in their feces and pass on the pathogen by contaminating food and water sources [10]. A primary example of an asymptomatic carrier who caused high transmission rates is "Typhoid Mary." Mary Mallon, an Irish cook in the New York City area, was the first known typhoid carrier in the United States. Despite her complete lack of symptoms, it is believed that Mallon caused a minimum of seven outbreaks of typhoid fever, resulting in 57 cases of the disease and 3 deaths [11].
Infectious dose, incubation, and colonization
Salmonella Typhi lacks acid tolerance, so a high infectious dose of 103 to 106 bacilli is needed for infection. Since it cannot survive in acidic conditions, a large portion of the bacteria are destroyed as they pass through the stomach, which can have a pH as low as 1-2. For this reason, there must be enough bacteria to overcome the condition of the stomach to successfully infiltrate the host [6]. After first encountering a large enough dose of Salmonella Typhi, the first symptoms will not appear until 7-14 days have passed.
To colonize, S. Typhi adheres to the mucosal lining of the small intestine and penetrates the epithelial cells. The bacteria spread to the peripheral lymphoid organs during secondary infection. The gallbladder serves as the primary reservoir of chronic infection. The formation of biofilms in the gut and on gallstones is a critical factor in the carriage and shedding of S. Typhi [12].
Epidemiology
Typhoid fever has continued to be a persistent threat in developing countries such as India, South American, Southeast Asia, and Africa due to the poor sanitation conditions and having no access to clean water [13]. 80% of typhoid cases are believed to have originated from Bangladesh, India, China, Indonesia, Nepal, Laos, Vietnam, or Pakistan [14]. The CDC reported that 22 million people are affected by Salmonella enterica serovar Typhi yearly, which resulted in approximately 200,000 deaths [15]. The United States reports between 200 and 300 of these cases yearly. Approximately 80% of the cases in the U.S. are a consequence of traveling to countries with a high occurrence of typhoid fever [16]. Before the use of antibiotics in the United States, the fatality rate was between 9-13%. However, modern day medicine has decreased this rate to just below one percent. For patients that receive no treatment, the mortality rate remains greater than 10 percent [3].
The World Health Organization claims that statistics are difficult to verify due to lack of proper supplies and measuring techniques in regions throughout Africa. However, they believe that the rate of typhoid fever infections has been gradually declining over the past several years in countries such as India and Chile. These results are attributed to increased attention towards health and sanitation methods [17].
Virulence Factors
Salmonella pathogenicity islands, SPI, are responsible for the majority of the virulence factors of the bacterium. Most of the effector molecules associated with the pathogen’s virulence are encoded from the Salmonella pathogenicity islands. For example, after entering a host cell, Salmonella Typhi will secrete effector proteins including SIPA and SptP. SIPA and SptP will alter the actin cytoskeleton of the host cell, which is responsible for cell migration [6].
SPI7 is the most important pathogenicity island because it codes for the Vi antigen that is expressed on the cells surface. The Vi antigen resides within a polysaccharide capsule, which is essential for increased virulence and severity of symptoms. Some believe that the polysaccharide capsule prevents lipopolysaccharide recognition by Pattern Recognition Receptors (PRRs) to avoid immune response, however, more research is needed. Secretion of the protein invasin allows non-phagocytic cells to ingest the bacterium permitting intracellular access that leads to the inhibition of oxidative leukocytes and rendering the innate immune response ineffective. Other possible factors include ion transporters, fimbriae, and flagella that are required for attachment and colonization [6].
Chimeric A2B5 typhoid toxin has made recent headlines. It is made up of two subunits including PltA and CdtB. PltA and CdtB are comparable to cytolethal and perussis toxins. This toxin is the cause of high fevers during the first and second week of infection [18].
Clinical Features
Symptoms of the infection can be classified according to a time frame after exposure. During the incubation period of 7 to 14 days, the patient is often asymptomatic as the bacteria colonizes and breaches the intestinal wall. However, within 72 hours after the onset of the illness, the patient may experience a high fever between 103-104°F.
A rash on the abdomen or chest, fatigue, dry cough, and diarrhea also characterizes this first week of infection. If the patient has not received treatment by the second week, symptoms will increasingly worsen. If diarrhea or constipation persists, extreme weight lost can occur. By the third week with no medical attention, the patient will become delirious and experience severe exhaustion, which known as the typhoid state. During this week severe complications may occur in association with prolonged infection of typhoid fever [19]. Approximately three percent of people with typhoid fever develop a perforated intestine causing internal bleeding which may cause blood to appear in the stool [4]. Perforation results in a hole in the small or large intestine triggering symptoms of abdominal pain, nausea, vomiting, and blood sepsis. Other complications associated with typhoid fever include myocarditis, intravascular coagulation, kidney or bladder infections, and meningitis. Psychiatric problems for such as delirium, hallucinations, and paranoid psychosis can also occur as a result of infection [19]. If the patient is able to survive to the fourth week, they will gradually improve and are able to regain their mental state. However, this recovery period may last up to several weeks or months [5]. Statistically, around 10 percent of patients will experience a relapse of symptoms after they have recovered. The probability of relapse is shown to increase with antibiotic usage. Three to five percent of survivors may also become long-term asymptomatic carriers with the potential to trigger new outbreaks [20].
These effects can be more severe or prolonged in children and the elderly. Bacteremia, or the spread of the pathogen into the blood stream, generally occurs in 5-10% of cases and can lead to more severe symptoms such as meningitis and infections of the bones and joints. This can be especially dangerous in immunocompromised patients such as those suffering from HIV or malaria [6].
Diagnosis
Stool cultures are the main form of diagnosis for this disease, but often times blood cultures are used instead. This is because stool cultures are best when collected in the early and late stages of the illness. When a blood culture is taken it is tested on MacConkey and EMB agar to get the results. [18] [21].
Salmonella Typhi enters the bloodstream upon infection and is carried by white blood cells to the liver, spleen, and bone marrow where multiplication occurs before they enter back into the bloodstream. As the bacteria invade the lymphatic tissue of the bowel, they proliferate. The bacteria then enter the intestinal tract and can be used in the diagnosis of stool cultures from the laboratory. In the early and late stages of the disease, stool cultures are more sensitive to culturing, but should be tested simultaneously with blood culture to make a definitive diagnosis. Additional testing may be done to differentiate between the different serovars of S. enterica species. These molecular biological tests are based on different antigens on the bacteria's surfaces, such as O, K, and H [3].
Treatment
Antibiotic therapy, specifically the use of ciprofloxacin and ampicillin, is the only effective treatment for this disease. For pregnant women, ceftriaxone is used for the safety of the fetus. Recently, antibiotic resistance by S. Typhi has increased and developed into a more serious issue concerning the effectiveness and use of antibiotics. To stimulate recovery, fluids and a healthy diet can be administered in addition to antibiotics [21]. Previously, antibiotic treatment for typhoid fever included regimens of ampicillin, trimthoproim-sulfamethoxazole, and chloramphenicol. Usage of these drugs is now limited due to developing drug resistance over the past twenty years. Strains specific to South America have shown significant resistant to antibiotic therapy. Currently, quinolone, macrolide, and third-generation cephalosporin antibiotics are used to treat resistant strains. Quinolone sensitivity has steadily declined in different parts of the world, but it remains significant in the United States [13].
To treat the carrier state, prolonged use of antibiotics is prescribed. Additionally, the direct site of chronic infection can be removed, such as gall bladder removal [22].
Prevention
Frequent handwashing and consuming only treated water is encouraged to avoid infection. Also, boiling water and properly handling raw fruits and vegetables can also decrease the risk of infection [22]. In association, two vaccines are available: inactivated typhoid vaccine administered via injection and live typhoid vaccine administered orally. Neither vaccine is 100% effective, and both require repeated immunizations [15].
To prevent reinfection while recovering from typhoid, one should avoid handling food, isolate personal items, wash hands often, and clean toilets, door handles, and telephone receivers daily [22].
Host Immune Response
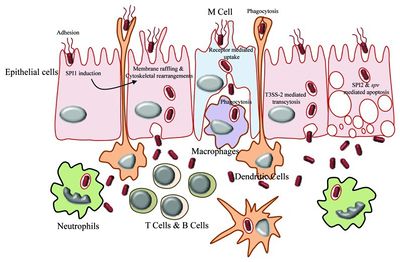
Salmonella enterica serovar Typhi can cause life-threatening bacterial infections called typhoid fever. The uncontrolled activation of the host innate immune response can potentially lead to systematic inflammation, tissue injury, intravascular coagulation, and even death [12].
Salmonella enterica serovar Typhi is an invasive pathogen. It is recognized by the host’s immune system using toll-like receptors (TLRs), which initiate the innate immune response. The TLRs recognize pathogen-associated molecular patterns (PAMPS) located on the surface of the pathogen. This recognition allows for the innate immune system to initiate its response, causing the activation and recruitment of macrophages, neutrophils, and cytokines. For example, IFN-γ is a significant cytokine for macrophage activation and early host resistance of S. Typhi [12].
Victims of typhoid fever are susceptible to reinfection because of the initial severe disruption of the gut microbiome. A typical host contains a microbiome of 1x1014 bacteria with an average of 500 to 1,000 different species. A healthy microbiome can protect the host’s epithelial cells from infection. The gut microbiome produces toxic metabolites that can suppress the virulence of S. Typhi’s gene expression, boost the host’s immune response, and help clear the intestinal lumen after non-typhoidal diarrhea. Additionally, apoptosis can strengthen the host’s defense by allowing the body to prevent further release of pro-inflammatory cell mediators. The ability of S. Typhi to use a microbiome nutrient, ethanolamine, allows it to colonize in the intestinal tract. This rich nutrient often allows S. Typhi to outcompete other pathogens. Antimicrobial treatment for S. Typhi may cause depletion of the host’s gut microbiome, which can lead to prolonged effects of intestinal colonization, and an increase in carrier status and fecal shedding [12].
Damage Response Framework
When Salmonella Typhi first enters the body there are no immediate symptoms. The usual incubation period of the disease is between 7-14 days, but some do develop a fever after about 72 hours. If not treated, symptoms will continue to worsen, and by one week, most patients will have a noticeable rash, cough, diarrhea, and will be fatigued. If no treatment was administered by the third week, one might enter what is called the typhoid state. This is characterized by extreme exhaustion, and for a small percentage of people, complications such as internal bleeding due to a perforated intestine may occur [19]. If left untreated, this disease is often fatal. However, if potent antibiotics are prescribed there is almost immediate relief. There would still be an incubation period, but if the medications were given shortly after the fever developed, symptoms would begin to subside about 24 hours after taking them. Usually the antibiotics are taken for 7-14 days to completely clear the disease, and if taken correctly, the patient should be back to a normal state of health after the two weeks. However, if they are not taken as prescribed, then typhoid fever can relapse [24].
References
1 University of Oklahoma Faculty and Staff.
2 Kenyan Waterborne Disease Center
3 "Salmonella Typhi" Salmonella Typhi.
4 "Salmonella enterica SPP" Public Health Agency of Canada.
5 "Typhoid fever- symptoms." NHS.
6 Brusch, J.L. "Typhoid Fever" Medscape.
7 Roumagnac, P., Weill, F.X., Dolecek, C., et al. "Evolutionary history of Salmonella typhi." Science.
8 Garai, P., Gnanadhas, D.P., Chakravortty, D. "Salmonella enterica serovars Typhimurium and Typhi as model organisms." US National Library of Medicine.
9 Deng, W., Liou, S., Plunkett, G., et al. "Comparative Genomics of Salmonella enterica Serovar Typhi Strains Ty2 and CT18." US National Library of Medicine.
10 Gopinath, S., Carden, S., Monach, D. "Shedding light on Salmonella carriers." Trends Microbiology.
11 "Mary Mallon (Typhoid Mary)." American Journal of Public Health.
12 de Jong, H.K., Parry, C.M., van der Pol, T. "Host-Pathogen Interaction in Invasive Salmonellosis" PLOS Pathogens.
13 Klochko, A. "Salmonellosis Treatment and Management." Medscape.
14 Chau, T.T., Campbell, I.J. "Antimicrobial Drug Resistance of Salmonella enterica Serovar Typhi in Asia and Molecular Mechanism of Reduced Susceptibility to the Fluoroquinolones." NCBI.
15 "Typhoid Fever" Center for Disease Control and Prevention.
16 Hohmann, E.L., "Epidemiology, microbiology, clinical manifestations, and diagnosis of typhoid fever" UpToDate
17 Crump, J.A., Luby, S.P., Mintz, E.D., "The global burden of typhoid fever" WHO
18 Song, J., Gao, X., Galan J.E. "Structure and Function of the Salmonella Typhi chimaeric A2B5 typhoid toxin" Nature: International weekly journal of science.
19 "Diseases and Conditions: Typhoid Fever" Mayo Clinic.
20 "Typhoid Fever." WebMD.
21 Pollack, D.V. "Salmonella enterica Typhi." University of Connecticut.
22 Balentine, J.R. "Typhoid Fever." Medicine Net.
23 "Typhoid Fever." Medline Plus.
24 "Salmonella enterica Serovar Typhimurium." PLOS
Created by Taylor Caswell, Sarah Grebennikov, Mary Kate Lowe, and Paige Whitson
Edited by Victoria Moss and Sarah Steckler
Students of Dr. Tyrrell Conway, University of Oklahoma
