Sexual Reproduction of Pathogenic Eukaryotes
Overview
By Kiersten Bell
Eukaryotic microbes do not simply reproduce through binary fission as bacteria do; rather, a large number of eukaryotic microbes undergo some form of sexual reproduction. Sex appears to be ubiquitous among larger eukaryotes, but its presence among the smaller, unicellular organisms is yet to be determined. Discovering sexual reproduction in eukaryotic microbes presents its own host of problems due to the fact that microbial sex is often rare, furtive, or cryptic [1]. Rare because many microbes reproduce in a sexual manner rarely and asexually commonly, furtive because many times sexual reproduction occurs under conditions which cannot be seen, or cryptic meaning that it can be observed but difficult to recognize what is going on [1]. Sexual reproduction is defined as any process in which chromosomes from two different cells, or two nuclei from the same cell, combine into a single nucleus and undergo homologous recombination to create a new genome [1, 2]. Sexual reproduction allows alleles to reassert, increasing genetic diversity and the removal of mutations, eventually resulting in progeny better fit to the environment [3].
Two modes of sexual reproduction exist in eukaryotic microbes: meiotic sex and parasexual cycles [1]. Meiotic sex is the reduction of a diploid cell to a haploid state and the eventual unification of two haploid cells to create a diploid zygote. A parasexual cycle begins with the fusion of two haploid cells and the fusion of the nuclei to create one diploid nucleus, where homologous recombination occurs. However, the diploid nucleus soon becomes unstable and loses random chromosomes, re-forming a haploid cell. Additionally, sexual reproduction can be either obligate or facultative [4]. A wide variety of eukaryotic microbes undergo sexual reproduction; however, research has centered around pathogenic eukaryotes due to the fact that parasitic reproduction can have a large effect upon the effectiveness of vaccines and treatment plans [1,5].
Finding Evidence for Sexual Reproduction
With eukaryotic reproduction being rare, furtive, and/or cryptic finding evidence of sexual reproduction occurring presents many problems. Many times it is assumed that if evidence of sexual reproduction cannot be found, an organism is reproducing asexually, which may or not be true. Additionally, sexual reproduction can occur in a laboratory setting, but it may never actually occur naturally. Evidence for sexual reproduction comes in a variety of forms: confirmed sexual interactions, direct evidence for sexual interactions, and indirect evidence that inconclusively suggests sex is occurring [6]. A confirmed sexual interaction is irrefutable proof of a change in the ploidy of an organism followed by restoration of the normal ploidy. Direct evidence is provided when observation of either reduction or restoration of the ploidy, as well as confirmed presence of meiosis genes. Indirect proof is provided by any number of observations: evidence of homologous recombination, cytoplasmic fusion, confirmation of a complex life cycle, or production of potential reproductive cells [6]. One manner of indirect proof is evidence of genes homologous to those in other organisms that are involved with sexual reproduction. Two such types of these genes exist: the Mating Type Locus (MAT) and genes involved with meiosis [3, 7, 8].
Mating Type Locus (MAT)
This locus has been discovered in a number of eukaryotic microbial species and controls mating type specificity. In the organism Cryptococcus neoformans the MAT locus expresses two mating types: a and α [8]. This locus occurs in two large iodiomorphs, two versions of the same gene that show low levels of homology, and encodes an estimated 20 genes, which are involved with mating and meiosis. The mating type determines which pheromones to produce and which pheromones in the environment to respond to, the necessary proteins encoded by this locus [8].Additionally, in the organism C. neoformans the locus encoding the α version of the gene has been linked to a high level of virulence than a cells due to the fact that α cells preferentially cross the blood-brain barrier. However, this is not always the case and in other species or some populations of C. neoformans, the α and the a cells are equally virulent [8].
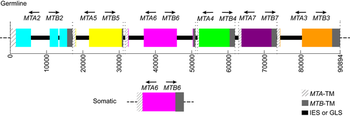
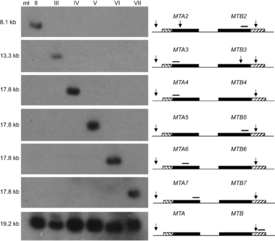
The MAT locus also does not necessarily only encode two mating types, in fact it has been shown to encode seven mating types in Tetrahymena thermophila through the interaction of two alleles [7]. Seven mating types are derived from the interaction of two alleles through programmed genome rearrangement. At the MAT locus, each allele contains six incomplete mating type gene pairs arranged head-to-head, figure 1. Each mating type gene is split into two parts separated by an exon. During formation of the somatic nucleus, the locus undergoes rearrangement to create a complete mating type gene and deletes the sequences encoding other potential mating type genes. The deletion of other mating types was confirmed by southern blot analysis, offering proof that only one mating type gene pair persists in the somatic nucleus of the future cells, figure 2. Thus, a complete mating type is created from the seven potential mating types. The deletion of the other potential mating type sequences ensures the mating type is permanent for each progeny cell. However, what mating type is selected is seemingly random and each progeny cell created will express a different mating type. T. thermophila presents one variation of the myriad of possibilities that exist in encoding mating types in eukaryotes [7].
The presence of this locus in the genome of a microbe represents the possibility that the microbe reproduces sexually. If the MAT locus can be amplified, the nucleotide sequence can provide preliminary clues to the reproductive nature of the organism. If the microbe reproduces asexually, if has no need to differentiate between two mating types, and MAT will be under the influence of relaxed selection. Mutations will collect over time until MAT is no longer functional. However, if an organism reproduces sexually, the mating type genes must continue working, and purifying selection will occur: mutation in MAT will confer a loss of fitness and the mutation will fail to be passed along to subsequent generations [9]. Based upon this analysis, decisions about whether to continue looking for a sexually reproductive state can be made. With evidence of purifying selection, further analysis will be needed to accumulate enough direct evidence for proof of sexual reproduction.
Meiosis Genes
The discovery of genes that are homologs of genes known to encode proteins involved in meiosis can provide preliminary direction on whether or not to continue searching for evidence of sexual reproduction. For example, the Giardia genome contains seven homologs of meiosis-specific genes, but meiosis has never been witnessed in this organism. Instead, this organism has a more complicated life cycle. The presence of these genes is being used to attempt to determine how genetic variation is controlled in this organism (see below). The presence of these homologs suggests that although meiosis may not occur, similar control could be, or the last common ancestor of this genus utilized meiosis during its life cycle [8].
Double Peaks
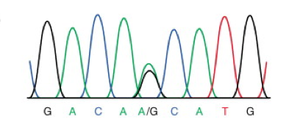
The search for evidence of sexual reproduction in Giardia populations is ongoing. The presence of genes involved in meiosis was discovered, and with this initial piece of evidence, the gene sequences were compared. The sequences showed considerable variation and showed clear evidence of recombination [5]. A later study examined allelic sequences more broadly, specifically looking for evidence of allelic sequence heterogeneity (ASH) [10]. The theory behind this search is that mutations will accumulate in genetic sequences differently in asexual and sexual reproduction. If an organism is reproducing asexually, mutations will occur at random throughout the chromosomes; additionally, it is much more rare for the same mutation to arise in the same gene. Thus mutations will accumulate in each chromosome and the mutations will not match each other. However, if crossing over is occurring in some form, the mutations will come to match each other due to the dual nuclei and tetraploidy existing in this species. By amplifying and sequencing the genome of an isolated line, a picture of alleles can be gained, figure 3. If a “double peak” persists in the genome of the lineage from a single cell this means that a mutation has arisen in the genome resulting in a heterozygous allele. This heterozygosity has been maintained through homologous recombination at some point in the sexual reproduction of this organism.
Population Genetics
Older methods of discovering evidence for sexual recombination are general population genetics studies. Burt et al. utilized DNA sequence-level polymorphisms, or different sequences encoding the same gene, as a test for evidence of sexual reproduction in Coccidiodes immitis [11]. This study revealed molecular evidence for recombination in this fungus due to the fact asexual and sexual reproduction result in different linkage patterns. The pattern obtained in this study was one characteristic of a haploid populations reproducing sexually. Prior to this study no evidence for a sexual interaction had been found [11].
Additional genetic evidence for sexual reproduction in eukaryotic microbes can arise from examining allelic frequencies. If asexual reproduction occurs, linkage disequilibrium results, but sexual reproduction will result in linkage equilibrium due to genetic recombination. Linkage disequilibrium is the non-random association of alleles at one or more loci, that may or may not be on the same chromosome, while linkage equilibrium is alleles found in the expected proportions. Linkage disequilibrium provides evidence for asexual reproduction- alleles will always be inherited together due to lack of sexual recombination, and linkage equilibrium provides evidence for sexual reproduction. For example in a series of populations of Cryptococcus gattii analyzed for the MATα locus there was strong evidence of asexual reproduction, but there was also unambiguous evidence of recombination. Thus, frequently C. gattii reproduces asexually, but sexual reproduction resulting in recombination occurs as well. This study also resulted in limited evidence of gene flow amongst different populations across India [12].
Another analysis compared the rates of mutation in nuclear DNA and mitochondrial DNA. Because DNA in the mitochondria is not subjected to homologous recombination during sexual reproduction, mutations should arise at a different rate than they will arise in nuclear DNA; however, in asexual reproduction, this discrepancy should not occur. Nuclear and mtDNA should be replicated at similar rates and mutations should arise randomly in both at a similar rate. Phylogenies can be created for both nuclear and mitochondrial DNA: if the phylogenies match asexual reproduction is occurring, but if the phylogenies differ, sexual reproduction is occurring. In this study, discrepancies resulted between the phylogenies, suggesting that sexual reproduction is occurring [13].
These more roundabout analyses are necessary because of the inherent differences in sexual reproduction in eukaryotic microbes and other eukaryotic organisms. In both of the above situations, the sexual reproduction has been labeled as cryptic [11,12]. This cryptic nature means that sexual reproduction is going to be much more challenging to recognize and define. Any evidence that can be accumulated makes classification of sexual reproduction more accepted. These analyses also show that these simpler beginning steps can still provide a strong starting point in further discovery of sexual reproduction.
Evolution of Sexual Reproduction
The widespread nature of sexual reproduction across eukaryotic lineages lends evidence to the conclusion that an ancient common ancestor of eukaryotic microbes had a lifestyle that required a sexual stage. Across eukaryotes, sexual mechanisms are similar for both transmission of genetic material as well as maintenance of genetic integrity, often epigenetic in nature. Although these similar processes exist, it is still unknown how sexual reproduction began and this remains a large question in the evolution of eukaryotes [2].
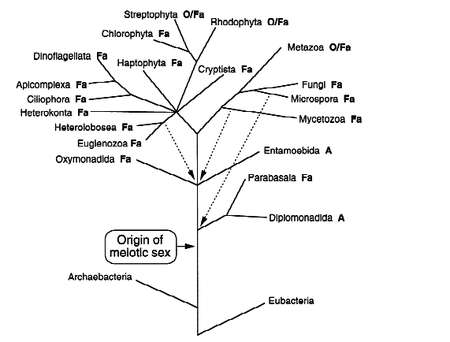
Within the question of the evolution of sexual reproduction comes the question of how obligate sexual reproduction arose from the facultative sexual reproduction common to most eukaryotic microbes that reproduce sexually, figure 4. Facultative sexual reproduction is asexual reproduction combined with infrequent rounds of sexual reproduction. The asexual reproduction allows microbes to exist in a stable environment without losing half of their genetic content each time they reproduce, potentially losing genes conferring fitness [6]. However, if the environment changes, the sexual phase allows mutations and new genes to arise, shuffling the genome to create new genotypes with an increased fitness to the changed environments. Facultative sexual reproduction allows adjustment to a slowly changing environment. It is still unclear how obligate sexuality arose from facultative sexuality [4, 14].
Sexual reproduction when compared to asexual reproduction comes with increased energetic and evolutionary costs. In larger eukaryotes, these costs center on the production and spread of male gametes as well as the cost of sexual behaviors in order to attract viable partners. However, these specific costs do not exist in eukaryotic microbes, which seemingly need to do neither of these things. The costs of reproducing sexually must be offset and result in increased fitness since so many microbial populations utilize this reproductive strategy [4].
Phylogenetic studies have been used to examine how sexual reproduction began and to determine if asexual microbes are representative of a more ancient condition or a recent loss. Two phylogenetic studies both argue that asexual microbes are a result of a recent loss, due to the fact that both sexual and asexual microbes align together throughout phylogenetic trees of eukaryotic microbes. In a study of the sexual reproduction or amoebas, varying levels of evidence of sexual reproduction were found spread throughout the eukaryotic tree of life, as well more focused amoebae trees [4]. Another phylogenetic study of the MAT locus in Penicillium and Talaromyces genuses saw a similar spread of reproductive types: sexual and asexual reproductive strategies spread throughout the tree. This study argues that asexual reproduction must represent a recent loss of sexual reproduction due to the presence of the MAT locus. The authors also claim that species that reproduce asexually are not able to persist long enough in the environment to adapt through mutation and diversify into many species. When environmental conditions are no longer optimal, sexual microbes can shuffle their genomes to obtain increased fitness; however, asexual microbes cannot most likely resulting in their death.
Examples of Sexual Reproduction in Eukaryotic Microbes
Sexual Reproduction in eukaryotic microbes can be difficult to characterize and represent in an accurate manner. Some methods of sexual reproduction are relatively simply following a strategy based upon meiosis to reduce the ploidy and karyogamy to reestablish the original ploidy, while other strategies involved center around alternation of generations similar to that found in plants. Alternation of generations begins in either a haploid or diploid state where asexual clonally occurs until environmental conditions change and prompt a shift to the other ploidy. Haploid cells fuse to form zygotes and create diploid cells while diploid cells undergo meiosis, undergoing homologous recombination in the process, to form haploid cells [5].
Following are some more bizarre examples of well-characterized reproductive strategies. In many cases recent discoveries are constantly increasing and changing the understanding of sexual reproduction across the eukaryotic world. Study of eukaryotic microbes usually focuses upon parasites. These organisms currently present the largest threat and an increased understanding of these pathogens will hopefully result in better vaccines and therapies [15].
Candida albicans
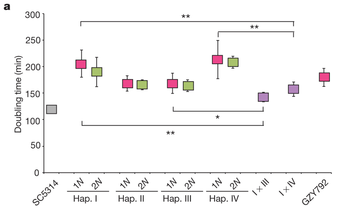
C. albicans is the most prevalent human fungal pathogen currently known. Until recently it was known that C. albicans reproduced in a parasexual cycle involving the fusion of two diploid cells to produce a tetraploid cell. This tetraploid cell then undergoes a reduction of the number of chromosomes to restore the diploid state. This reduction is not through meiosis, but rather a chromosomal loss mechanism, resulting in a high degree of aneuploidy as well as restoration of the diploid state [3]. It was recently discovered that C. albicans also forms mating-competent haploid cells. These cells were located through flow cytometry profiles and subsequent amplification of the MAT locus, based on this analysis it was concluded that haploid cells arise through the same chromosomal loss at a rate of 1-3 per 100,000 cells. It was also noted that the haploid cell lines grew slower than either an established diploid line or a diploid line arising from the fusing of two haploid cells. This growth pattern is expected if the diploid state results in a masking of lethal alleles that would slow growth in a haploid state, figure 5.
Haploid cells will then switch from the white to the opaque state, necessary for mating to occur, and mate with cells of the opposite mating type, determined by the MAT locus, to form diploids. There is also evidence that the diploid state can result from a failure of cytokinesis after mitosis. C. albicans represent a unique organism that has the ability to form and utilize a variety of ploidy states to best adapt to the environment and increase genetic diversity. For example, potentially lethal mutations as a result of inbreeding can be complemented with a functional allele in this reproductive mechanism [16].
Giardia
Giardia species are intestinal pathogens of mammals and is a significant cause of diarrheal disease around the world, figure 6. Not only is this pathogen widespread, but it additionally spreads antibiotic resistance through its many species [17]. The genus Giardia encompasses protists, which have two diploid nuclei and eight flagellae [1].
In 2007 it was discovered that Giardia lamblia isolates from three distinct lineages had extremely low genetic diversity in both coding and noncoding regions, proposing that the distinct patterns of genetic variation found resulted from the presence of genetic exchange in some form [17]. In addition, another study found the presence of five homologs of genes necessary for meiosis [5]. Thus, it can be inferred that some kind of exchange of genetic material is occurring, but whether this is representative of sexual reproduction cannot be determined without more direct evidence.
Later studies determined that Giardia species have a very distinct life cycle consisting of two stages. The first state, trophozoites inhabit the intestine of the host. Here, imbedded in the intestinal lining the trophozoites reproduce clonally. From here, the organisms can take one of two pathways—continue to reproduce clonally or form a cyst to be excreted into the environment. Another host animal will then consume such a cyst and once inside the intestinal lining of a new host, transform back into a trophozite, beginning binary fission and starting the cycle again [1].
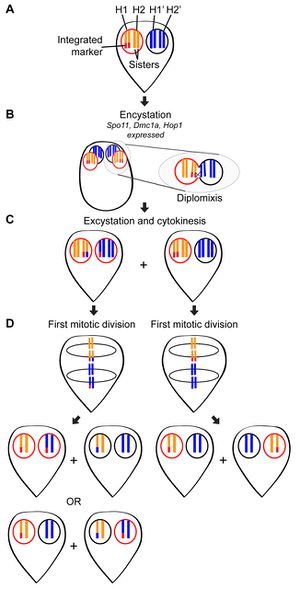
A recent study by Carpenter et al. in 2012 found direct evidence of genetic exchange without meiosis in Giardia intestinalis. In cyst formation, four neuclei are created from the two that are present in the trophozite. Though immunofluorescence staining, it was discovered that cysts are formed though an incomplete mitotic division. Instead of cytokinesis, the cell internalizes its flagella as the cyst wall finishes formation [8]. Within the nuclei of the cyst it is believed that genetic exchange occurs during diplomixis [10]. Diplomixis is the fusing of two nuclei, leading to an exchange of genetic material between the two nuclei [10]. This genetic recombination is believed to result in the low levels of genetic diversity witnessed in a variety of populations, figure 7 [8]. An additional finding was the discovery that during excystation and reformation of trophozies each daughter cell has a copy of each parental nucleus [8]. Although much is known and understood about the way that Giardia reproduces, still much is unknown. The 2012 study ended with a list of the many things still to be discovered so that the spread of drug resistance is can be halted or countered [8].
References
1- Birky, C.W. 2009. Giardia Sex? Yes, but how and how much? Trends in Parasitology 26: 70-74.
2- Parfrey, L.W., D.J.G. Lahr, and L.A. Katz. 2008. The Dynamic Nature of Eukaryotic Genomes. Mol. Biol. Evol. 25: 787-794.
3- Heitman, J. 2010. Evolution of Eukaryotic Microbial Pathogens via Covert Sexual Reproduction. Cell Host & Microbe: Review. 8: 86-99.
4- Xu, J. 2005. Cost of Interacting with Sexual Partners in a Facultative Sexual Microbe. Genetics. 171:1597-1604.
5- Logsdon, J.M. 2008. Evolutionary Genetics: Sex happens in Giardia. Current Biology 18: R66-R68.
6- Lahr, D.J.G., L.W. Parfrey, E.A.D. Mitchell, L.A. Katz, and E. Lara. 2011. The chastity of amoebae: re-evaluating evidence for sex in amoeboid organisms. Proc. R. Soc. B.: 2081-2090.
7- Cervantes, M.D., E.P. Hamilton, J. Xiong, M.J. Lawson, D. Yuan, M. Hadjithomas, W. Miao, and E. Orias. 2013. Selecting One of Several Mating Types through Gene Segment Joining and Deletion in Tetrahymena thermophile. PLOS Biology.j 11(3): e1001518.
8- Carpenter, M.L., Z.J. Assaf, S. Gourguechon, and W.Z. Cande. 2012. Nuclear inheritance and genetic exchange without meiosis in the binucleate parasite Giardia intestinalis. Journal of Cell Science 125: 2523-2532.
9- López-Villavicencio, M., G. Aguileta, T. Giraud, D.M. de Vienne, S. Lacoste, A. Couloux, and J. Dupont. 2010. Sex in Penicillium: Combined phylogenetic and experimental approaches. Fungal Genetics and Biology. 47: 693-706.
10-Andersson, J.O. 2011. Double peaks reveal rare diplomonad sex. Trends in Parasitology 28: 46-52.
11- Burt, A., D.A. Carter, G.L. Koenig, T.J. White, and J.W. Taylor. 1996. Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proc. Natl. Acad. Sci. 93: 770-773.
12- Chowdhary, A., S.S. Hiremath, S. Sun, T. Kowhik, H.S. Randhawa, and J. Xu. 2011. Genetic differentiation, recombination and clonal expansion in environmental populations of Cryptococcus gattii in India. Environmental Microbiology. 13: 1875-1888.
13- Ramírez, J.D., F. Guhl, L.A. Messenger, M.D. Lewis, M. Montilla, Z. Cucunba, M.A. Miles, and M.S. Llewellyn. 2012. Contemporary cryptic sexuality in Trypanosoma cruzi. Molecular Ecology. 21: 4216-4226.
14- Dacks, J. and A. Roger. 1998. The First Sexual Lineage and the Relevance of Facultative Sex. J Mol Evol 48: 779-783.
15- Heitman, J. 2006. Sexual Reproduction and the Evolution of Microbial Pathogens. Current Biology 16: R711-725.
16- Hickman, M. A., G. Zeng, A. Forche, M.P. Hirakawa, D. Abbey, B.D. Harrison, Y. Wang, C. Su, R.J. Bennett, Y. Wang, and J. Berman. 2013. The ‘obligate diploid’ Candida albicans forms mating-competent haploids. Nature. 000: 1-6.
17- Teodorovic, S., J.M. Braverman, and H.G. Elmendorf. 2007. Unusually Low Levels of Genetic Variation among Giardia lamblia Isolates. Eukaryotic Cell 6: 1421-1430.
