Streptococcus equi subsp. equi
Characteristics of the symbiont/pathogen
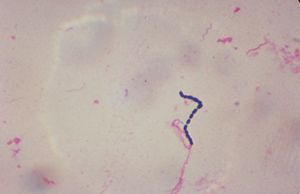
The pathogen involved in this symbionic relationship Streptococcus equi. The bacterium S. equi subsp. equi infects Equus caballus, the horse, and causes a disease called strangles [10].
S. equi is a gram positive bacterium. Irregularly shaped cocci are typical of the species. Its incubation period is 3-14 days. The bacterium, which is in Lancefield’s group C, also causes red blood cells to lyse [10]. The bacterium can be grown on blood agar at 37°C under aerobic conditions. The bacteria form β-haemolytic colonies, which appear mucousy with no pigment. They are catalase-negative and facultative anaerobes. In a study to determine species of an unknown bacterium, certain strains and other species of the Streptococcus genus were looked at. In this study, Fernandez et. al. looked at S. equi subsp. ruminatorum subsp. nov., but they also looked at general features of the S. equi species including other strains (subsp. equi and subsp. zooepidemicus). The scientists found several characteristics in the different species that distinguish them from each other, and they also found some characteristics unique to S. equi [2]. S. equi subsp. equi is thought to have evolved from S. equi subsp. zooepidemicus, which is usually harmless. Only S. equi subsp. equi actually causes Strangles, though [4].
S. equi produces a protein called SeM, which increases its virulence. The protein binds fibrinogen and immunoglobulin G in order to inhibit the deposition of C3b. This causes phagocytes to be destroyed. The protein contains a sub-typing region (the region in which alterations of amino acid codes are present), a fibrinogen binding region, and an IgG binding region [8].
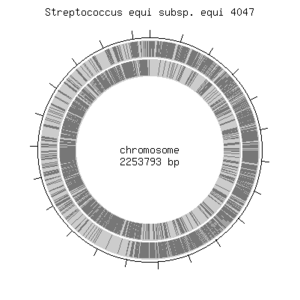
The genome of S. equi contains 2253793 base pairs and contains circular DNA. The complete genome of the species has been discovered [4].
Characteristics of the host
The host of the bacterium Streptococcus equi subsp. equi is the horse, Equus caballus. The horse is an herbivore and its ancestors, the earliest of the Equidae family which horses are from, are known to have originated 45- 55 million years ago. About five million years ago, the modern equus species was born. Early horses ate leaves, but as the teeth evolved, they became grass eaters [12]. Ovis aries, the sheep, and Capra hircus, the goat, are two species which host other subspecies of the Streptococcus equi species [2].
The genome of the horse contains about 2,680,000,000 base pairs. It includes 64 chromosomes, including 31 pairs of autosomal chromosomes and the sex chromosomes. There is much known about the horse genome and it is completely mapped out [1].

Host-Symbiont Interaction
The interaction is pathogenic. Streptococcus equi subsp. equi is a parasite to the horse as the bacterium survives and thrives while the horse suffers dramatically from its effects. The host acquires the disease called strangles form this bacterium. Strangles has many symptoms including a high fever, usually the first sign. The horse will develop nasal discharge. Lymph nodes can become very enlarged and abscesses will form in the submandibular and retropharyngeal lymph nodes. Lymph nodes will become swollen and painful after about one week of infection. These abscesses drain for several days or even weeks. The name "Strangles" actually came about because the enlarged lymph nodes of the horse obstruct the airway and cause suffocation in some cases. "Bastard strangles" occurs when the bacterium spreads to other organs of the thorax and abdomen [10].
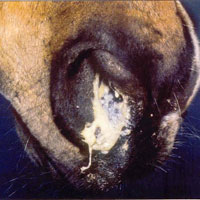
Shedding of the bacteria begins one to two days after the horse has developed symptoms. The bacteria is shed through nasal discharge for two to three weeks in most cases. Transmission of the bacteria can be direct or indirect. It can be spread horse-to-horse or from contaminated objects. Contaminated barns, water troughs, clothes, tack or feed can spread the bacteria. Horses that seem healthy can actually incubate the disease and spread it, but only later develop symptoms themselves. Horses recovering from Strangles can still shed the bacteria for prolonged periods of time [10].

The relationship is facultative since Streptococcus equi subsp. equi is a pathogen, the horse does not benefit from the interaction at all. The bacteria can also sometimes survive without the horse under favorable conditions.There is little research performed on the ability of Streptococcus equi to survive outside the horse host. Weese et. al. found that very few bacteria survived even a few days on outdoor rubber, wood and metal surfaces [11]. Another study showed that the organism can survive 63 days on wood at two degrees Celsius and 48 days on glass or wood at 20 degrees Celsius. It does not survive well with other bacteria from soil [7].
Molecular Insights into the Symbiosis
S. equi enters the mouth or nose of the horse and attaches to the epithelial cells in the tonsils. Surface proteins SzPSe, Se73.9 and Se51.9 are known to assist in binding of the bacterium to the epithelial cells. The bacterium then spreads to the lymph nodes. Peptidoglycan of the cell walls of the bacterium cause neutrophils, or white blood cells, to come to the sight. The hyaluronic capsule, SeM protein (antiphagocytic), and Mac protein of S. equi inhibit the neutrophils ability to phagocytose the bacteria. In other words, there are several proteins that are antiphagocytic, so neutrophils cannot attack and kill the bacteria. Here, S. equi is able to flourish. Streptolysin S and streptokinase damage cell membranes. This causes cells to lyse and is thought to lead to abscesses [10].
IgA and IgG are antibodies in horses which attack the S. equi bacteria. These are the two significant antibodies that help the horse fight the bacterial infection [3].
Ecological and Evolutionary Aspects
Streptococcus equi subsp. equi is derived from Streptococcus equi subsp. zooepidemicus. Several important genes involved with S. equi pathogenesis are also found in S. pyogenes, a bacteria affecting humans. Streptococcus equi subsp. equi or Se4047 contains an inversion from other Streptococcus species at about 14 kbp. This gene codes for capsule production, indicating why S. equi has high levels of hyaluronate capsule. S. equi has many pseudogenes, which are genes that are no longer expressed, which indicates that the bacterium has recent loss and gain of genes. Se4047 contains 58 partially deleted genes and 78 pseudogenes, much more than S. equi subsp. zooepidemicus.There is also a deletion at 5 kb, which affects carbohydrate metabolism. Se4047 also lacks genes that enable it to ferment lactose, sorbitol or ribose. Se4047 has developed hyaluronate lyase gene different than that of other Streptococcus species, which explains high levels of hyaluronate capsule in Se4047. This enzyme could explain why Se4047 rarely extends past the lymphatic system and. Since levels of this hyaluronate lyase are low, there are high levels of hyaluronate, increasing resistance to phagocytosis. This also decreases adhesion to the mucosal surface. Another one-base deletion (fne gene) causes lower levels of fibronectin, which increases virulence of the pathogen. A nonsense mutation at codon 43 produces longer pili, which increase collagen-binding, allowing it to infect its host more easily. Another important part of the evolution of the bacterium is the four prophage in Se4047. These prophage transport genes, contributing to the bacterium's ability to survive and adapt to its environment. These are the most significant mutations of Se4047 that make it different from other Streptococcus species, but it is known that these mutations are the only differences between S. equi subsp. equi and S. equi subsp. zooepidemicus. This supports the idea that S. equi subsp. equi is derived from S. equi subsp. zooepidemicus [4].
Disinfection and sterilization will destroy the bacterium, but detergents will not. There are no other environmental factors known to regulate the symbiosis [5]. Some horses will develop more complications from the bacteria and young horses are more susceptible, probably due to having less exposure and immunity, but environmental factors are not known to affect the interaction [10].
Recent Discoveries
In a recent study in 2010, Ivens et. al. investigated whether a surface protein SeM in Streptococcus equi subsp. equi caused the disease, strangles. They amplified the variable region of the SeM gene from S. equi isolates using PCR and sequenced the genes. They were able to conclude that the disease differed based on SeM allele sequences [6].
Another recent study looked at the haem-uptake system in S. equi. It was found that S. equi, S. pyogenes and S. zooepidemicus have closely-related haem-uptake systems. S. equi homologues were analyzed and it was determined that S. equi has a Shr protein that is not enveloped. The bacterium also contains other factors that result in erythorcyte lysis and release of hemoglobin and haem, which are iron sources for the organism. This iron source assists the bacterium in its ability to begin infection within its host. They found that similarities between the uptake systems of S. equi and S. zooepidemicus support the theory that S. equi is derived form S. zooepidemicus [9].
References
[1] Chowdhary, B.P. and Raudsepp, T. 2008. The horse genome derby: racing from map to whole genome sequence. Chromosome Research 16:109-127.
[2] Fernandez, E., Blume, V., Garrido, P., Collins, M.D., Mateos, A., Dominquez, L., and Fernandez-Garayzabal, J.F. 2004 Streptococcus equi subsp. ruminatorum subsp. nov., isolated from mastitis in small ruminants. International Journal of Systematic and Evolutionary Microbioogy 54:2291-2296.
[3] Galan, J.E. and Timoney, J.F. 1985. Mucosal nasopharyngeal immune responses of horses to protein antigens of Streptococcus equi. Infection and Immunity 47:623-628.
[4] Holden, M.T.G., Heather, Z., Paillot, R., Steward, K.F., Webb, K., Ainslie, F., Jourdan, T., Bason, N.C., Holroyd, N.E., Mungall, Kl, Quail, M.A., Sanders, M., Simmonds, M., Willey, D., Brooks, K., Aanensen, D.M., Spratt, B.G., Jolley, K.A., Maiden, M.C.J., Kehoe, M., Chanter, N., Bentley, S.D., Robinson, C., Maskell, D.J., Parkhill, J., and Waller, A.S. 2009. Genomic evidence for the evolution of Streptococcus equi: host restriction, increased virulence, and genetic exchange with human pathogens. PLoS Pathogens 5:1-14.
[5] Hoopes, J.T., Stark, C.J., Kim, H.A., Sussman, D.J., Donovan, D.M., and Nelson, D.C. 2009. Use of bacteriophage lysin, PlyC, as an enzyme disinfectant against Streptococcus equi. Applied and Environmental Microbiology 75:1388-1394.
[6] Ivens, P.A.S., Matthews, D., Webb, K., Newton, J.R., Steward, K., Waller, A.S., Robinson, C., and Slater, J.D. 2010. Molecular characterisation of 'strangles' outbreaks in the UK: The use of M-protein typing of Streptococcus equi subsp. equi. Equine Veterinary Journal 43:359-364.
[7] Jorm, L.R. 1992. Laboratory studies on the survival of Streptococcus equi subsp. equi on surfaces. From Plowright, W., Rossdale, P.D., and Wade, J.F. 1992. Equine infectious diseases VI: Proceedings of the sixth international conference, 7-11 July 1991. R and W Publications 39-43.
[8] Kelly, C., Bugg, M., Robinson, C., Mitchell, Z., Davis-Poynter, N., Newton, R., Jolley, K.A., Maiden, M.C.J., and Waller, A.S. 2006. Sequence variation of the SeM Gene of Streptococcus equi allows discrimination of the source of strangles outbreaks. Journal of Clinical Microbiology 44:480-486.
[9] Meehan, M., Burke, F.M., Macken, S., and Owen, P. 2010. Characterization of the haem-uptake system of the equine pathogen Streptococcus equi subsp. equi. Microbiology 156:1824-1835.
[10] Sweeney, C.R., Timoney, J.F., Newton, J.R., and Hines, M.T. 2005. Streptococcus equi infections in horses: Guidelines for treatment, control, and prevention of strangles. J Vet Intern Med 19:123-134.
[11] Weese, J.S., Jarlot, C., and Morley, P.S. 2009. Survival of Streptococcus equi on surfaces in outdoor environment. The Canadian Veterinary Journal 50:968-970.
[12] Wikipedia Horse
Edited by Brooke Reamer, students of Grace Lim-Fong
