Sulfolobus islandicus rod-shaped virus 2

By Andrew Van Horn
Introduction
Sulfolobus islandicus rod-shaped virus 2 (SIRV2) is a lytic double-stranded DNA archaeal virus that infects Sulfolobus archaea [1] [2]. SIRV2 falls into the greater taxonomy of the Rudiviridae family. Microbiologists have taken interest in SIRV2 due to its thermophilic and acidophilic properties needed to infect Sulfolobus. SIRV2 displays unique properties from many other viruses [1]. These include a unique viral release mechanism of which there are very few similar structures found in the natural world. Furthermore, SIRV2 is a lytic virus that acts by severely degrading host chromosomes. Additionally, SIRV2 is commonly at the forefront of the generally limited field of archaeal viral research [3][4].
Sulfolobus islandicus Archaeon
Sulfolobus islandicus is an archaeon commonly found in Icelandic sulfur hot springs [5]. S. islandicus is a member of the Sulfolobaceae family and the Sulfolobales order. Once more, S. islandicus is a member of the Crenaracheota kingdom of the phylum Archaea. S. islandicus thrives in extreme environments of acidic pH 3 and high temperatures around 80°C. Notably, the intracellular pH of S. islandicus is only slightly acidic, approximately pH 6. Additionally, S. islandicus metabolizes sulfur, though the methods can vary between strains and species [6]. Sulfur metabolism can be done through sulfur oxygenase/reductase and sulfur reductase, among others. However, the extensive methods of sulfur metabolism in S. islandicus are not fully understood.
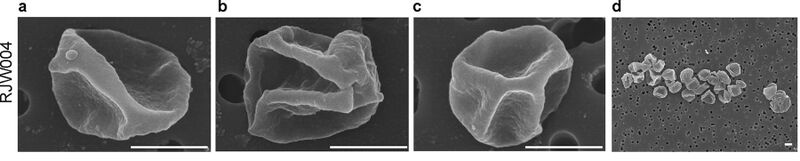
Pathogenicity
While believed until very recently to be a host-carrier virus that transmitted without lysing host cells[2], this has been revealed to not be the case[1]. In 2009, Bize et al. used flow cytometry to document large-scale chromosomal degradations and DNA damage that led to them recharacterizing SIRV2 as a cytocidal lytic virus. In S. islandicus cells infected with SIRV2, reduction in genome size was documented as early as half an hour after infection. After 12 hours, the majority of their cell populations did not contain any flow cytometry-detectable DNA. Interestingly, a slight increase in intracellular DNA of 1.3 Mb was detected around 3 hours after infection. Following the spike, decreases in intracellular DNA occurred. This spike was attributed to viral DNA replication that occurs quickly after infection.
The latent period of SIRV2 was determined by Bize et al. to be 8-10 hours[1]. After 8-10 hours, almost no host DNA was detectable. Additionally, virions are then released through Virus-Associated Pyramids (VAPs). Estimates of burst size for SIRV2 in S. islandicus are generally around 30-50 virions[1][2][7].
Bize et al. also showed that no virions were released before 8-10 hours after infection. Furthermore, cell death was associated with virion release at 8-10 hours by using a membrane potential sensitive probe.
Additionally, it was demonstrated that SIRV2 is able to superinfect S. islandicus. This means that a cell infected by SIRV2 is unable to be infected by other viruses while presently infected by SIRV2.
Genome Structure
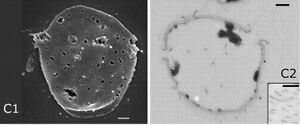
The SIRV2 genome is double-stranded DNA consisting of approximately 35.8 kbp[2]. By contrast, this is about 10% larger than the related SIRV1. Additionally, the DNA of SIRV2 forms a superhelix through the use of a 15.8-kD DNA-binding protein. Each turn of this DNA-protein superhelix complex is made of 16.5 turns of B-form DNA and is 4.3 nm long. It has been suggested that the genome of SIRV2 is condensed by a factor of 12.7. Moreover, the ends of the SIRV2 genome are not attached to proteins, rather, the ends are managed through covalent bonding into hairpin structures.
However, virion capsid proteins dissociated in solution can bind to the DNA helices and trigger a conformational shift from B-form DNA to A-form DNA[8].
In solution, around half of virion capsid proteins can unfold and become unstructured. These proteins can then attach to the B-form DNA to form a dimer. These dimers induce a conformational shift away to A-form DNA. Once bound to the DNA, the capsid proteins maintain an α-helical shape along the DNA. Once dimerized, the A-form DNA has an overall twist of 11.2 base pairs per turn. Additionally, this binding process is saturable by nature. Once a weight ratio of capsid protein to DNA is 3.5:1, no more capsid protein can bind to the DNA. This phenomenon of proteins binding to DNA in microbes is well-established in the process of endospore formation and bacterial cells responding to environmental stress[9]. As such, it has been suggested that shifting to A-form DNA through protein binding is a mechanism for microbes to protect their genomes against adverse conditions. SIRV2 likely utilizes this mechanism due to its extreme environment. The conformational shift reduces solvent passage to DNA and in turn exposure to extreme conditions. It should be noted, however, that homology between the bacterial and SIRV2 protein-binding to shift from B-form to A-form DNA has been refuted by sequence analysis, suggesting convergent evolution of these mechanisms.
It should also be noted that despite the great diversity and lack of homologous traits among archaeal viruses, major nucleocapsid proteins (MCPs) are homologous between SIRV2 and other archaeal viruses[10].
Interestingly, when comparing the MCPs of SIRV2 to Acidianus filamentous virus 1 (AFV1) and Pyrobaculum filamentous virus 2 (PFV2). The secondary structures of MCPs in SIRV2, AFV1, and PFV1 display the same flexible secondary structure alignment. This phenomenon led to the discovery of homology between the MCPs. Interestingly, the MCPs of AFV1 and PFV2 form heterodimers with the DNA while the MCPs of SIRV2 form homodimers. Despite this, the MCPs are exceptionally similar in their biological functions of covering A-form DNA.
Viral Life Cycle
Infection
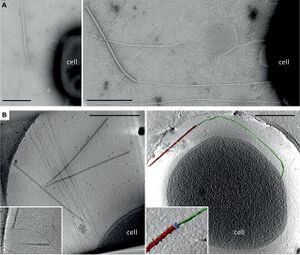
SIRV2 binds to filaments protruding from the surface of S. islandicus to reach the cell for infection [11]. SIRV2 virions use their tail fibers to bind the tips of long filaments on the cell surface. Virions then migrate down the length of the filament until reaching the cell surface. Upon surface-contact, the virions begin to dissemble and release their genome into the new host cell. Approximately three virions can be bound to an individual filament at a given time. However, only two virions can be bound at a given time to the tip of the filament. Other virions can simultaneously be migrating down the length of the filament towards the cell surface. Virions show an initial binding preference for the tips of the filaments in their initial attachment. Fortunately, filaments are highly abundant on the S. islandicus cell surface. Once virions are bond to the filaments or cell surface, they are irreversibly bound.
Moreover, the rate of infection by SIRV2 of S. islandicus is extremely high[11]. In Quemin et al. 2013, they demonstrated that approximately 80% of virions injected to a culture of S. islandicus were absorbed within thirty seconds, and almost all virions were absorbed after approximately 25 minutes. This rapid absorption is likely facilitated at least in part by the great abundance of filaments for virions to bind. Furthermore, the ability of multiple virions to bind to each filament allows for a rapid infection process. This rapid infection process combined with a lengthy latent period of 8-10 hours has led scientists to suggest that this cycle timing is highly evolved for the extreme conditions in which S. islandicus, and thus SIRV2, live[1][11]. If living in an unfavorable environment of Icelandic hot springs in which the temperatures are greater than 80°C and as acidic as pH 3, it is favorable for SIRV2 to reduce exposure to the extreme environment by minimizing infection time and increasing the time spent within the relative safety of S. islandicus.
Replication
SIRV2 replicates its genome by a unique mechanism that involves localizing replication and using host cell proteins [12].
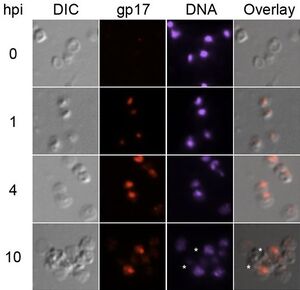
SIRV2 creates a replication focus near the terminus of the host cell. Viral DNA localizes to one end of the cell for replication. Furthermore, three proteins have been identified to localize in the replication focus: viral ssDNA binding protein gp17, host cell proliferating nuclear antigen (PCNA), and DNA polymerase I (Dpo1). Interestingly, Sulfolobus encodes four DNA polymerases[13].
However, Dpo2, Dpo3, and Dpo4 do not play any consequential role in the viral replication of SIRV2[12]. As such, Dpo1 is accepted to be the polymerase involved in viral DNA replication. Simultaneously, host PCNA is also utilized by SIRV2. It is believed that SIRV2 uses PCNA because PCNA recruits and organizes the DNA replication and repair processes. In this sense, by recruiting PCNA, SIRV2 unlocks a variety of tools that it can use to replicate and protect its genome. During DNA replication, PCNA and Dpo1 are almost completely confined to the replication focus. Interestingly, Dpo1 and gp17 expression increase throughout infection. This suggests a mechanism by which SIRV2 is able to not only increase its own gene expression but alter the gene expression of the host cell.
The SIRV2 replication cycle is also unique in that in undergoes multiple diverse stages[14].
Replication begins with the binding of a replication initiating protein (Rep). Asymmetric strand-displacement replication then proceeds to make circular dimer intermediates. Displacement replication is a process of continuous DNA replication on both stands that results in a hairpin loop on the end of the slower (light) strand when the faster (heavy) strand reaches the origin of light strand replication[15].
This hairpin loop is then replicated by additional DNA polymerases in lagging strand replication. Once circular dimer intermediates have been synthesized, both strand displacement and strand-coupled replication occur together. Rolling circle replication then occurs once to produce multimers of ssDNA. Then multiple re-initiation events of DNA replication cause the DNA to form brush-like structures. These brush-like structures result from multiple strands of associated ssDNA that fan out. The brush-like structures then undergo coupled-replication to produce multiple new progeny dsDNA genomes.
Virion Release
SIRV2 employs one of the most unique viral release mechanisms seen in nature[7].
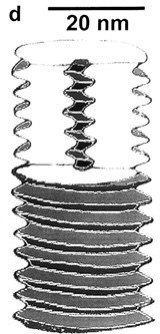
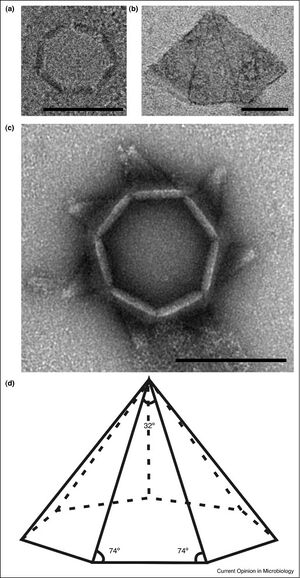
Encoded in SIRV2-ORF98 is the gene for SIRV2-P98, the protein constituent of the SIRV2 Virus-Associated Pyramid (VAP) that releases virions. After P98 synthesis, the proteins self-assemble in the cytoplasm into a seven-sided open-base pyramidal structure. This shape of VAP is exceptionally rare in nature, with only two other known proteins with a seven-fold structure in nature: the archaeal 20S proteasome and scallop muscle myosin filaments. The continued growth and synthesis of the VAP occurs along the base of each triangular face. As a result, there is a constant presence of growing VAPs of various sizes in the host cell’s cytoplasm.
To release virions, the VAP embeds itself in the membrane with its base facing inwards. Virions can then assemble in the opening forged by the VAP. Once enough virions have assembled near the VAP, the VAP undergoes a conformational shift to release the virions. The seven triangular faces open away from the center to create an open channel for virion release. Interestingly, the faces are curved in the open state. Additionally, the VAPs must grow to a certain size before they are capable of opening and releasing virions. A suggested size for functionality has been a minimum diameter of 250 nm.
When SIRV2-ORF98 has been placed into other organisms such as Escherichia coli the bacterial cells still produced the VAP. This demonstrated to researchers that the P98 proteins were able to self-assemble into the VAP. Moreover, the VAP inserted itself into the inner membrane of the bacterial cells. This finding was curious given biochemical differences between bacterial and archaeal membranes that often lead to protein insertion specificity.
Notably, the Sulfolobus turreted icosahedral virus (STIV) shows a similar viral release mechanism, though the VAP geometry maintains some key differences and is less stable.
Anti-CRISPR Defense Mechanisms
Sulfolobus islandicis is one of 85% of archaea that use CRISPR (clustered regularly interspaced short palindromic repeats) to defend against viruses [3][16].
Consequently, SIRV2 has evolved mechanisms to defend against CRISPR attack. These Anti-CRISPR (Acr) proteins inhibit the CRISPR defense mechanisms in S. islandicus. More specifically, they often inhibit Cas enzyme components of the CRISPR system.
The first Acr protein discovered in archaea, AcrID1, was discovered in SIRV2 in 2018[17].
The AcrID1 protein is a dimeric αβ-sandwich protein that shows great resistance to various pH values and temperatures. Found by infecting a mutant strain of S. islandicus carrying nonfunctional genes for the CRISPR-Cas subtypes I-A, I-D, and III-B with SIRV2. The researchers then isolated a mutant strain of SIRV2 carrying a 4 kb gene deletion. This deletion reduced infectivity greatly against wild type strains of S. islandicus containing functional genes for Cas subtypes I-A, I-D, and III-B. Then by introducing a gene to the mutant SIRV2 that produced an inhibitor of Cas subtype I-D, infectivity was restored. As such, AcrID1 is now characterized as an inhibitor of the Cas subtype I-D by inhibiting Cas10d. Cas10d is an important target-cleaving nuclease needed for the interference step of CRISPR response. By inhibiting Cas10d, the CRISPR system is far less effective in preventing SIRV2 infection, replication, and lysis/release. Additionally, more than 50 homologues of AcrID1 have been identified throughout four archaeal viral families that infect Sulfolobales: rudiviruses, lipothrixiviruses, fuselloviruses, and monocaudaviruses. Notably, 12 homologues of AcrID1 are located within the SIRV2 genome, though their functions are still poorly understood.
Another notable Acr protein in SIRV2 is AcrIIIB1. AcrIIIB1 is a crucial defense mechanism of CRISPR-Cas type III defense proteins. Type III defense is an ancient and highly conserved system throughout archaea and bacteria. In turn, type III Acr proteins are some of the most abundant throughout archaeal and bacterial viruses. Type III systems attack the viral genome by hybridizing crRNA to a complimentary protospacer in the viral genome. Cas enzymes are then recruited to cleave the viral genome before releasing the secondary messenger cyclic oligoadenylates (cOA). AcrIIIB1 interferes with the base-pairing of crRNA in CRISPR-Cas subtype III-B systems to the protospacers in the viral genome. More specifically, AcrIIIB1 has only been shown to provide protection for middle and later stage viral genes. Through conformational analysis of type III-B subtype proteins exposed to AcrIIIB1 it has been suggested the AcrIIIB1 interferes with the release of cOA by the CRISPR-Cas complex when hybridized to the viral genome. The interference of cOA release prevents the activation of Csx1, an accessory RNase required for the targeting of middle and late viral transcripts. These AcrIIIB1 interference mechanisms are highly conserved throughout the Rudiviridae and some Lipothrixviridae.
CRISPR-editing of the SIRV2 Genome
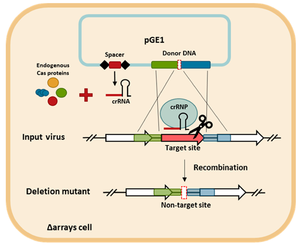
Viruses have been among the most difficult and last biological units to be modified through CRISPR-Cas genome editing[4]. Despite the difficulty of viral genome editing – and the lack of focus on arachael viruses throughout virology – SIRV2 was successfully edited using CRISPR for the first time in 2018. Furthermore, SIRV2 was the first archaeal virus to be edited by CRISPR.
The mechanism by which CRISPR editing was accomplished is methodologically fascinating. Mayo-Muñoz et al. 2018[4] utilized editing of the host genome to modify the genome of SIRV2. S. islandicus LAL14/1 were transformed to acquire a cloned plasmid containing CRISPR-edited genes. These genes included a spacer targeting the to-be-deleted gene, as well as two regions of DNA inserted to flank the spacer gene. After successful transformation, the S. islandicus were infected with SIRV2. During the viral replication, viral DNA recombination allowed the editied DNA to enter the viral particles. The DNA then caused a selective preservation of recombinant viruses. Non-recombinant viruses were killed by CRIPSR ribonucleoprotein (crRNP).
Nanotechnology
SIRV 2 is being investigated for potential biotechnological applications as a nanobuilding block[18].
Viral nanoparticles (VNPs) are often used in biotech due to their robust, rigid, modifiable, and diverse structures. Applications of VNPs are diverse, including nanoelectronics, nanofactories, magneto-storage, and biomedicine. Cowpea mosaic virus (CPMV) nanoparticles are commonly used to synthesize biotechnological materials. However, SIRV2 has been proposed as a potential alternative to the traditional CPMV. SIRV2 poses a unique opportunity to use an acidophilic and thermophilic VNP in biotech. Additionally, SIRV2 is stable in many conditions that would ordinarily destroy a viral particle. SIRV2 particles have been able to survive and maintain infectivity in hostile conditions such as 50% dimethyl sulfoxide (DMSO) 50% H2O for at least 6 days. This longevity is similar in ethanol/water solutions. As such, researchers are investigating mechanisms of chemical tagging and VNP utilization. SIRV2 viral particles contain many surface amino residues that are readily addressed, giving the opportunity for biotechnologists to equip the cell with unique amine groups, carboxy groups, and more [19].
It has also been demonstrated that SIRV2 viral particles have glycosylated surfaces equipped with oxidizable and modifiable surface carbohydrates[18]. These carbohydrates can be attacked by various compounds, including multiple forms of biotin. While biotin attacks label across the entire surface of the viral particles when attached to carboxy or carbohydrate, the biotins preferentially labeled the amines attached to tail fibers of SIRV2 particles. The selectivity of amine labeling by biotins has excited scientists as they pursue further research into assembly of VNPs like SIRV2 particles.
To date, little has been synthesized with SIRV2 nanoparticles[20]. SIRV2 nanoparticles are still in the proof-of-concept stage of development. Nonetheless, scientists and biotech companies are excited by the potential of these unique VNPs.
Conclusion
SIRV2 is an example of the great diversity seen in archaeal viruses. SIRV2 has properties that characterize it as unique from even the most distinct viruses known today. Most famously, SIRV2 uses a seven-sided open-bottom VAP to release virions from a host cell. Furthermore, it only does so once the chromosomes of the host have been degraded. This organization of virion release is unique and unparalleled in the virosphere.
SIRV2 is able to survive at extreme heat and acidity through specific adaptions like their DNA coiling. As such, SIRV2 has become a focal point of archaeal virus research. Acr defense systems have been further understood by SIRV2 research, as well as how scientists can edit lytic archaeal virus genomes with CRISPR. Moving forward, these unique properties will continue to be explored by biotechnologists. SIRV2 shows great potential as a nanoparticle that could lead to important applications in the future.
While much is evidently known about SIRV2, there is still much to learn and better understand. Archaeal viruses are poorly understood and understudied by the scientific community. The lack of archaeal pathogens towards humans has resulted in archaea, and in turn their viruses, often remaining under-investigated. However, the exciting properties and potential applications of SIRV2 likely pave the way for future research in both the academic and corporate sectors.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Bize A, Karlsson EA, Ekefjä Rd B K, Quax TEF, Pina M, Prevost M-C, Forterre P, Tenaillon O, Bernander R, Prangishvili D. 2009. A unique virus release mechanism in the Archaea.
- ↑ 2.0 2.1 2.2 2.3 Prangishvili D, Arnold HP, Götz D, Ziese U, Holz I, Kristjansson JK, Zillig W. 1999. A Novel Virus Family, the Rudiviridae: Structure, Virus-Host Interactions and Genome Variability of the Sulfolobus Viruses SIRV1 and SIRV2.
- ↑ 3.0 3.1 Peng X, Mayo-Muñoz D, Bhoobalan-Chitty Y, Martínez-Álvarez L. 2020. Anti-CRISPR Proteins in Archaea.
- ↑ 4.0 4.1 4.2 Mayo-Muñoz D, He F, Jørgensen JB, Madsen PK, Bhoobalan-Chitty Y, Peng X. 2018. Anti-crispr-based and crispr-based genome editing of sulfolobus islandicus rod-shaped virus 2. Viruses 10.
- ↑ Lewis AM, Recalde A, Bräsen C, Bräsen B, Counts JA, Nussbaum P, Bost J, Schocke L, Shen L, Willard DJ, Quax TEF, Peeters E, Siebers B, Albers S-V, Kelly RM. 2021. The biology of thermoacidophilic archaea from the order Sulfolobales. FEMS Microbiology Reviews 063:1–60.
- ↑ Dai X, Wang H, Zhang Z, Li K, Zhang X, Mora-López M, Jiang C, Liu C, Wang L, Zhu Y, Hernández-Ascencio W, Dong Z, Huang L. 2016. Genome sequencing of sulfolobus sp. A20 from costa rica and comparative analyses of the putative pathways of carbon, nitrogen, and sulfur metabolism in various sulfolobus strains. Frontiers in Microbiology 7.
- ↑ 7.0 7.1 Prangishvili D, Quax TEF. 2011. Exceptional virion release mechanism: One more surprise from archaeal viruses. Current Opinion in Microbiology.
- ↑ Cusanovich DA, Daza R, Adey A, Pliner HA, Christiansen L, Gunderson KL, Steemers FJ, Trapnell C, Shendure J. 2015. Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science (1979) 348:910–914.
- ↑ Mohr SC, Sokolov NVHA, He C, Setlow P. 1991. Binding of small acid-soluble spore proteins from Bacillus subtilis changes the conformation of DNA from B to A (CD/Fourier-transform infrared spectroscopy/DNA photochemistry/UV resistance)Proc. Natl. Acad. Sci. USA.
- ↑ Wang F, Baquero DP, Su Z, Osinski T, Prangishvili D, Egelman EH, Krupovic M. Structure of a filamentous virus uncovers familial ties within the archaeal virosphere.
- ↑ 11.0 11.1 11.2 Quemin ERJ, Lucas S, Daum B, Quax TEF, Kühlbrandt W, Forterre P, Albers S-V, Prangishvili D, Krupovic M. 2013. First Insights into the Entry Process of Hyperthermophilic Archaeal Viruses. Journal of Virology 87:13379–13385.
- ↑ 12.0 12.1 Martínez-Alvarez L, Deng L, Peng X. 2017. Formation of a Viral Replication Focus in Sulfolobus Cells Infected by the Rudivirus Sulfolobus islandicus Rod-Shaped Virus 2.
- ↑ Choi JY, Eoff RL, Pence MG, Wang J, Martin M v., Kim EJ, Folkmann LM, Guengerich FP. 2011. Roles of the four DNA polymerases of the crenarchaeon Sulfolobus solfataricus and accessory proteins in DNA replication. Journal of Biological Chemistry 286:31180–31193.
- ↑ Martínez-Alvarez L, Bell SD, Peng X. 2016. Multiple consecutive initiation of replication producing novel brush-like intermediates at the termini of linear viral dsDNA genomes with hairpin ends. Nucleic Acids Research 44:8799–8809.
- ↑ Miralles Fusté J, Shi Y, Wanrooij S, Zhu X, Jemt E, Persson Ö, Sabouri N, Gustafsson CM, Falkenberg M. 2014. In Vivo Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication. PLoS Genetics 10.
- ↑ Makarova, K.S., Wolf, Y.I., Iranzo, J. et al. Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol 18, 67–83 (2020).
- ↑ He F, Bhoobalan-Chitty Y, Van LB, Kjeldsen AL, Dedola M, Makarova KS, Koonin E v., Brodersen DE, Peng X. 2018. Anti-CRISPR proteins encoded by archaeal lytic viruses inhibit subtype I-D immunity. Nature Microbiology 3:461–469.
- ↑ 18.0 18.1 Evans DJ. 2009. Exploitation of plant and archaeal viruses in bionanotechnology, p. 665–670. In Biochemical Society Transactions.
- ↑ Steinmetz NF, Bize A, Findlay KC, Lomonossoff GP, Manchester M, Evans DJ, Prangishvili D. 2008. Site-specific and spatially controlled addressability of a new viral nanobuilding block: Sulfolobus islandicus rod-shaped virus 2. Advanced Functional Materials 18:3478–3486.
- ↑ Narayanan KB, Han SS. 2017. Helical plant viral nanoparticles - Bioinspired synthesis of nanomaterials and nanostructures. Bioinspiration and Biomimetics. Institute of Physics Publishing.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College
