Telaprevir (VX-950) a novel antiviral treatment for Hepatitis C virus patients
Introduction

Hepatitis C virus (HCV) is a positive-strand RNA virus with a genome of about 10,000 nucleotides, displaying significant heterogeneity, with six main genotypes and more than 50 subtypes (Schinazi et al. 2010). The host range of the virus is restricted to hepatocytes, liver cells (Horscroft et al. 2005). The HCV epidemic is a serious health threat, infecting over 170 million people worldwide (Lin et al. 2005). Moreover, three to four million people are newly infected each year (Ressink et al. 2006). The infection is often asymptomatic in its early stages, but the majority of HCV-infected individuals develop chronic hepatitis over time, which eventually advances to liver scarring (cirrhosis), and liver cancer (hepatocellular carcinoma) (Moriishi and Matsuura 2007) (Figure 2.). The epidemiology of the virus is not well understood. However, it is known that HCV is transmissible through sexual contact and blood-to-blood contact. In the United States, blood-to-blood transmission of HCV is greatest among injecting drug users (IDUs), but the virus the virus can also be acquired through blood transfusions, and contact with other sources of infected blood product (Simmonds 2000).
Standard care for Hepatitis C patients: Interferon alfa-2b plus ribavirin
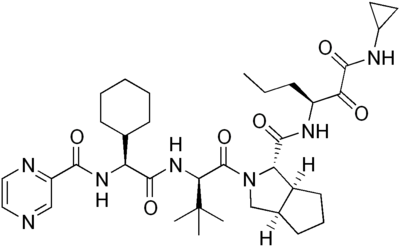
Since 1998, standard care for patients infected with hepatitis C has been weekly injections of interferon alfa-2b combined with daily oral administration of ribavirin (Keeffe 2006). Interferons function by disrupting viral replication through immune response stimulation. More specifically, they activate immune cells, such as natural killer cells and macrophages; they increase recognition of infection by up-regulating antigen presentation to T lymphocytes; and they increase the ability of uninfected host cells to resist new infections (Hunt 2009). The treatment’s other component, ribavirin, is a pro-drug which when metabolized resembles purine RNA nucleotides. In this form, it interferes with the RNA metabolism required for viral replication. The mechanism of interference is not well understood (Feld and Hoofnagle 2005). Side effects of the drug range from mild flu-like symptoms to severe symptoms like hair loss, bloody stools, depression and suicidal tendencies (AHFS 2010). While the interferon/ribavirin treatment's sustained response rates are 54-56%, these results imply that 40-50% of patients do not have lasting improvements with treatment (Feld and Hoofnagle 2005). Pharmaceutical companies are developing alternative HCV treatments in attempt to improve care for infected individuals. One such candidate drug is Telaprevir (VX-950) co-developed by Vertex and Johnson & Johnson (Figure 1).
Telaprevir (VX-950), a novel antiviral treatment for Hepatitis C
Challenges of HCV anti-viral drug discovery and development
The types of infectious virus cell assays that were and are routinely available for drug development against other viruses, like HIV, are not widely available as drug discovery tools in HCV research. With respect to animal models, chimpanzees are the only animals capable of being infected with HCV. Consequently, no small robust animal model exists in which to study HCV infections. Thus, scientists rely on a combination of antiviral activity in HCV replicon cell culture and pharmacokinetic profiling in animals as proxy indicators of telaprevir’s efficacy before evaluating the drug in clinical trials (Lin et al. 2006).
Replicon cell culture system
One of the milestones in anti-HCV drug discovery was the development of a non-infectious HCV replicon system by Lohmann and colleagues. In the replicon system, modified forms of HCV genome are able to replicate to high levels in human hepatoma cells (Huh-7) and stably transfected replicon cell lines are established after continuous drug selection. Replicons contain the viral 5' and 3' untranslated regions. Internal ribosome entry sites (IRES) direct translation to proceed from the first cistron to the second cistron (locus) of the modified genome. The first cistron encodes a reporter gene, eg. luciferase, to allow detection of RNA replication in infected cells; the second cistron translates the HCV non-structural proteins required for viral replication. Replicons of this description are termed bi-cistronic. Mono-cistronic replicons have also been developed. They typically consist of a selectable marker, to detect replication, with a cleavable sequence fused directly to the non-structural proteins. This system became a widely used cell-based assay for determining the structure-based activity relation (SAR) of inhibitors of HCV replication, like telaprevir. Before investigating telaprevir's mechanism of action in depth, it is useful to review basic information about the HCV genome and the virus's replication cycle (Horscroft et al. 2005).
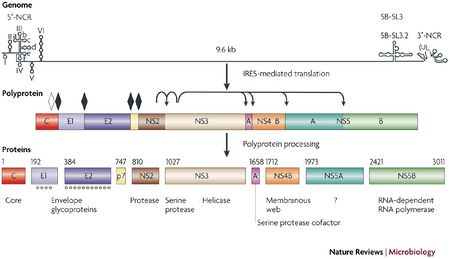
HCV genomic structure
HCV is classified in the Hepacivirus genus within the Flaviviridae family. HCV has a positive strand RNA genome of about 10,0000 nucleotides. Its genome contains a 5’ non-coding region (NCR), which includes an internal ribosome entry site (IRES), a large open reading frame that encodes at least 10 structural and non-structural proteins, and a 3’-NCR. The structural proteins, which form the virion, include the core protein and the envelope proteins, E1 and E2. The non-structural proteins include the p7 ion channel, the NS2-3 protease, the NS3 serine protease and RNA helicase, the NS4A polypeptide, the NS4B and NS5A proteins and the NS5B RNA-dependent RNA polymerase (RdRp) (Figure 3; Moradpour et al. 2007.)
HCV replication cyle
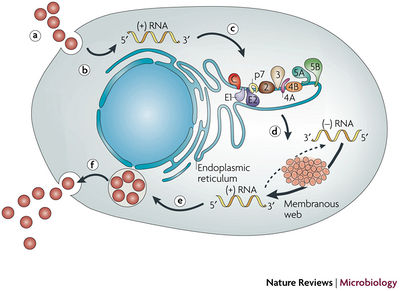
Figure 4. provides an overview of the lifecycle of hepatitis C virus (HCV):
a. Virus binding and internalization-
The receptor molecule to which HCV binds is unknown, but several candidate molecules have been proposed: CD81, a membrane protein that is found on the surface of many cell types, including hepatocytes, the LDL receptor (LDLR), scavenger receptor class B type I (SR-BI), and claudin-1. After binding to the host cell receptor, HCV enters the cell by clathrin-mediated endocytosis, by passage through endosomal, low pH compartments and presumably endosomal membrane fusion.
b. Cytoplasm release and uncoating-
After the genome is released into the cytosol, uncoating takes place; very little is known about the uncoating process.
c. IRES-mediated translation and polyprotein processing-
Translation initiation occurs in a binary complex that forms between the IRES and the 40 S ribosomal subunit. This complex develops further to form full membrane-associated replication compartments. In the compartments, translation and protein processing take place. During the polyprotein processing, cellular and viral proteases process both mature structural and non-structural proteins. The structural proteins and the p7 polypeptide are processed by the endoplasmic reticulum (ER) signal peptidase, whereas the non-structural proteins are processed by two viral proteases, the NS2-3 protease, and the NS3-4A serine protease. The NS3-4A serine protease is the target molecule of telaprevir.
d. Packaging and assembly-
NS2 and possible other non-structural proteins that have yet to be defined are involved in this step of replication.
e. Virion maturation and release-
Virions presumably form by budding into the ER, or an Er-derived compartment, and exit the cell through the secretory pathway.
f. The topology of HCV structural and non-structural proteins at the endoplasmic reticulum membrane (Moradpour et al. 2007).
A video representation of HCV replication cycle can be viewed by clicking the link below:
http://www.youtube.com/watch?v=eI5t0PbgnBk
Target molecule: NS3-4A serine protease complex
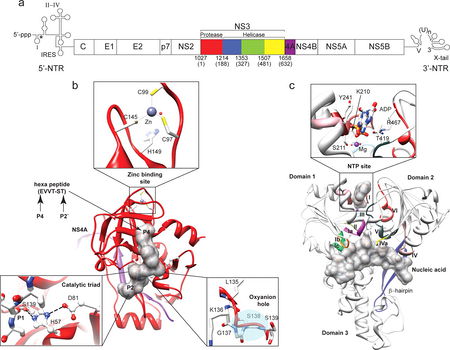
The conformation of HCV NS3 serine protease interacting with the NS4A co-factor peptide is the target molecule of telaprevir (Clercq 2007). NS3 protease is a serine protease that belongs to the trypsin/chymotrypsin protease superfamily. The enzyme consists of two ß-barrel domains that are flanked by two short ∂-helicase (Figure 5b Raney et al. 2010). One of the ß-strands of the N-terminal ß-barrel originates from the central hydrophobic region of NS4A. The structure is stabilized by a Zn2+ ion that is coordinated by three cysteine residues and one water molecule (Figure 5b Raney et al. 2010). An oxyanion hole (backbone amides of Gly-137 and Ser-139) and a catalytic triad (Ser-139, His-5, and Asp-81) are required for proper protease function (Figure 5b Raney et al. 2010). The NS4A cofactor helps establish the proper positioning of the catalytic triad and the substrate (NS5A/5B), playing an important role in catalytic efficiency and substrate specificity. The NS3-4A complex is responsible for the proteolytic cleavage at 4 junctions in the nonstructural protein region of the HCV polyprotein precursor. Moreover, it has recently been shown that the NS3-4A serine protease cleaves and thereby inactivates two crucial adaptor proteins in innate immune sensing, namely Triff77 (aka TICAM-1) and Cardif78 (aka MAVS79, IPS-1 and VISA81) (Moradpour 2007).
By inhibiting the action of NS3-4A, telaprevir halts the formation of essential nonstructural proteins, and in turn reduces the number of virulent viral particles formed during the replication cycle. Additionally, it prevents NS3-4A induced inactivation of important adaptor proteins in the immune system, decreasing the pathogenesis of the virus (Melnikova 2008).
Telaprevir's mechanism of action: protease inhibition
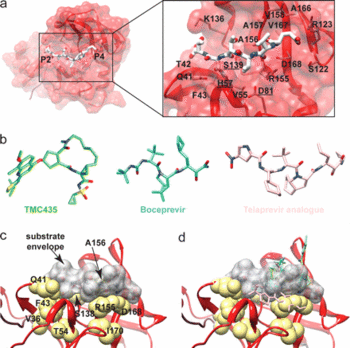
Telaprevir is a NS3-4A protease inhibitor that competes with the substrate, NS5A/5B, and targets the substrate-binding site, the serine residue of the protease’s catalytic triangle (Figure 5b and 6b). Upon binding to the protease, the ∂-ketomide (ketone and amide group of telaprevir) forms a reversible covalent linkage with Ser-139 (Figure 6d). Because of this mechanism, telaprevir is often referred to as an ∂-ketomide-based “serine trap” inhibitor.
Although the drug development process is incredibly involved and time consuming, it is useful to understand, on a basic level, the development of telaprevir. The evolution of ∂-ketomide protease inhibitors is based on the structure of the NS5A/5B substrate and the product-based inhibition of the NS3-4A protease. Both approaches resulted in the same structural activity relationship (SAR). Initially, these kinds of inhibitors were studied using scaffold proteins. Scaffold proteins are key regulators in many cell-signaling pathways and are used as biological indicators. In the case of ∂-ketomide inhibitors, scaffold proteins indicate how well compounds are inhibiting normal protease signaling pathways. From numerous potentially inhibiting compounds, a “lead compound”, the compound with the greatest efficacy, is selected. Next, the compound is “optimized”. Certain aspects of the compounds structures are altered to increase its efficacy. In the optimization process of telaprevir's development, the P1’ amide substituent was optimized first; mainly the steric (steric number refers to the number of atoms bonded to the central atom) and charge (negative or positive) requirements were changed to increase the affinity of the molecule to its binding site. P2, P3, P4 substituents (functional groups in the middle of the telaprevir compound) were also optimized, following the same criteria (steric and charge requirements) as the P1’ amide substituent optimization.
After its discovery and optimization, telaprevir was assessed and given a preclinical profile. The binding of telaprevir to the NS3-4A serine protease is thought to occur in at least 2 stages. The first stage, telaprevir binds weakly to the protease to form a impermanent “collision” complex, a complex where two reactive molecules are held together weakly by van der Waals forces. This complex is slowly rearranged to form a more tightly bound, covalent complex. The covalent bond is formed between Ser-139 and the ∂-ketomide (ketone/amide group on the N-terminus of the peptide); this was confirmed by x-ray crystallography. The dissociation of the covalent complex is a slow process, with a half-life of 58 minutes. To complete telaprevir's preclinical profile, the specificity, potency, and toxicity of the compound was assayed using the cell replicon culture system. After researchers confirmed telaprevir's high specificity and potency and low toxicity, drug development was able to proceed into clinical trials (Lin et al. 2006).
Telaprevir (VX-950) clinical trials
More than 2,500 people with hepatitis C have received telaprevir-based regimes as part of Phase 2 studies and the Phase 3 ADVANCE, ILLUMINATE, and REALIZE clinical studies. These studies enrolled people with genotype 1 hepatitis C who had not been treated previously, or who had been treated and did not experience sustained viral response. To date, the telaprevir clinical development program is the largest conducted for any investigational direct-acting antiviral hepatitis C therapy (Vertex 2010).
Reviewed below are two clinical studies that aimed to evaluate some aspect, eg. antiviral activity, pharmacokinetics, and/or the safety, of telaprevir treatments in individuals infected with HCV.
Reesink et al.
Reesink et al. performed a phase I, placebo-controlled, double-blind study that evaluated the antiviral activity, pharmacokinetics, and safety of telaprevir (VX-950) alone. More specifically, the aim of the study was to evaluate the safety and tolerability of ascending multiple doses of VX-950 in patients with chronic hepatitis C. To be eligible patients: were male or female, between the ages of 18 and 65 years old, had body mass indexes (BMIs) between 18.5 and 29.0 kg/m2 (males) or 18.5 and 32.5 kg/m2 (females), had a HCV RNA level ≥1 X 105 IU/mL, had HCV genotype 1 (any subtype), and had an alanine aminotransferase (ALT) concentration ≤4 times the upper limit of normal. ALT is an enzyme found mainly in the liver and is used to assess liver damage incurred by HCV infection.
Three panels of patients that met the aforementioned criteria were enrolled. Panels 1 and 3 comprised of 10 patients on VX-950 and 2 on placebo. Panel 2 comprised of 8 patients on VX-950 and 2 on placebo. Patients were randomly assigned to VX-950 or placebo, both were administered as an oral suspension. For 14 consecutive days, patients received either 450 mg (panel 1) or 750 mg (panel 2) VX-950 every 8 hours (q8h), 1250 mg (panel 3) VX-950 every 12 hours (q12h), or a matching placebo regimen (Reesink et al. 2006).
The antiviral activity of VX-950 was assessed by measuring plasma HCV RNA levels. The drug’s pharmacokinetics was assessed by measuring the concentration of VX-950 in the plasma. Patients were monitored for safety and tolerability at regular intervals from the start of dosing through the experiments competition. Safety assessments included physical examinations and vital signs, clinical laboratory tests, 12-lead digital electrocardiograms (ECG), and questioning about adverse side effects (Reesink et al. 2006).
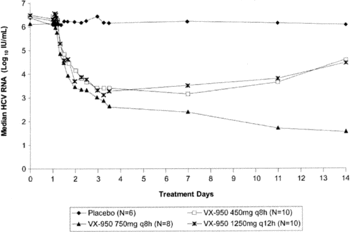
Reesink et al. found rapid and substantial viral decline in patients treated with VX-950 for fourteen days. The decline was experienced in two phases. In phase 1 (the first four days of dosing) the most substantial viral decline was observed. Phase 2 was characterized by a more gradual decline, and in some patients an increase in viral RNA. All patients treated with VX-950 had at least a 2-log10 decrease from baseline in HCV RNA. HCV RNA levels decreased steadily in the 450-g q8h and 1250-mg q12h groups until day seven. Between day 7 and 14, HCV RNA levels in these groups actually increased, demonstrating viral breakthrough. Reesink et al. hypothesized that this breakthrough was related to the selection of HCV variants with decreased sensitivity to VX-950 (Figure 7).
The median HCV RNA value decreased through the entire dosing period in group that took 750mg of the drug every eight hours. Moreover, HCV RNA decreased below the limit of detection (10IU/mL) in 3 patients: 1 in the 450-mg q8h group and 2 in the 750-mg q8h group. Three additional patients had HCV RNA levels that were below the lower limit of quantification (30 IU/mL) at day 14 (Reesink et al. 2006) (Figure 7).
Regarding the pharmacokinetics of the drug, VX-950 accumulated on multiple dosing with a median accumulation index of 1.8. The initial rapid decline in HCV RNA was related to maximal exposure to VX-950, and the second phase of viral decline was sustained by trough concentrations of the drug (Reesink et al. 2006). There were no severe or serious adverse side effects and no breaks in the dosing regime or discontinuations due to adverse events. The most frequent adverse events were headache, flatulence, diarrhea, frequent urination, dry mouth, fatigue, abdominal pain, nausea, back pain, dry skin, and common colds. No clinically significant laboratory results in hematology or clinical chemistry tests occurred in any of the patients. Furthermore, no patients had clinically significant changes in ECGs from the predosing baselines (Reesink et al. 2006).
Ultimately, VX-950 was effective in reducing viral load in a population in which 79% of patients had not responded to prior interferon based regimens (Reesink et al. 2006). Where interferon based treatments failed to achieve a lasting viral response, VX-950 was successful. Interestingly, clinicians and researchers have begun to study the effects of a combination treatment, part old interferon/ribavirin and part new VX-950. One such study was conducted by Forestier et al. who investigated the antiviral activity of telaprevir combined with peginterferon alpha-2a in patients with chronic hepatitis C. The experimental design and results are reviewed below.
Forestier et al.
Much of Forestier et al.’s study design and organization is the same as Reesink et al. The experiment’s participant criteria were the same as Reesink et al., with the addition that only individuals who had not previously been treated for hepatitis C were eligible to participate. The study was placebo-controlled for telaprevir; peginterferon alfa-2a treatment was open-labeled. (Forestier et al. 2007).
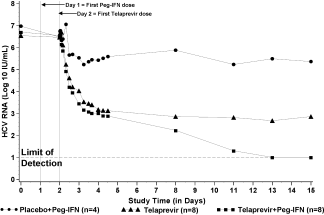
Like Reesink et al., Forrestier et al. observed viral decline in two phases. Overall, patients treated with telaprevir and interferon alfa-2a had greater decreases in HCV RNA and more sustained declines in comparison to patients with telaprevir only treatments. The median change in HCV RNA from baseline to day 15 was -1.09 log10 in the placebo and peginterferon alfa-2a group and -3.99 log10 in the telaprevir group. The greatest decrease in HCV RNA was in the telaprevir and peginterferon alfa-2a combination group, from baseline to day 15 RNA was -5.49 log10 (Figure 8. Forestier et al. 2007).
The second phase of decline was more sustained in the telaprevir and peginterferon alfa-2a group than in the telaprevir alone group. In the telaprevir group, 4 patients had continued decline, 2 patients had viral plateau, and 2 had viral rebound whereas, all patients in the telaprevir and peginterferon alfa-2a group had continued decline during the entire drug dosing period. In the telaprevir group, 1 patient had undetectable HCV RNA levels at day 15. In comparison, 6 patients in the telaprevir and peginterferon alfa-2a group were below the lower limit of quantification in HCV RNA levels, and 4 patients had undetectable HCV RNA levels (Figure 8. Forestier et al. 2007).
Clinical study conclusions
Taken together, the findings of Reesink et al. and Forestier et al. confirm that telaprevir significantly decreases HCV replication, as measured by viral RNA. Furthermore, these trials confirm that a sustained viral response can be obtained using telaprevir where the standard interferon alfa-2b plus ribavirin treatment had previously failed. Forestier et al. adds to the discussion the notion of combining new and old antiviral treatments (telaprevir and interferon alfa-2a), and presents a convincing study that supports the administration of a combined treatment for maximal antiviral activity.
Alternate drugs
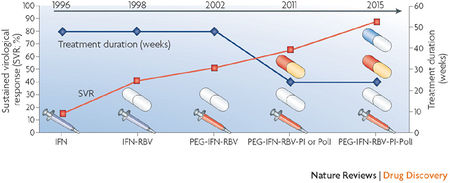
Telaprevir, VX-950 is one of over two dozen molecules being studied for their potential to supplement or replace either or both elements of the standard interferon/ribavirin combination treatment (Figure 9). Drug developers target various stages of HCV replication. The most successful inhibitors are protease inhibitor, which have been discussed in depth above, and polymerase inhibitors, outlined below.
Polymerase inhibitors-
Although the concept of polymerase inhibition for antiviral therapy has had much success with diseases like HIV, hepatitis B and herpes, developing a polymerase inhibitor for HCV has been difficult. Two lead compounds have been established, though, R1626 and R7128. Of the two, R7128 might have the more attractive profile because it has been well tolerated and produced rapid virological responses in treatment-naive patients (Melnikova 2008).
Other inhibitors include:
Entry inhibitors-
Entry inhibitors prevent HCV attachment and entry by interfering with HCV receptor molecules like CD81, Claudin 1, Scavenger receptor type b1, and Low density lipoprotein receptor. Several entry inhibitors are in the preclinical stage of development including: Sp-30, PRO 206, and REP 9C. The development of entry inhibitors is an emerging and exciting approach to treating HCV; however, until the specific mechanisms of action driving many of these compounds are elucidated, clinical trials will be delayed.
Helicase inhibitors-
The NS3 region of the HCV genome contains a serine protease that possesses NTPase and helicase activity. Together, the coupled NTPase and helicase unwind the double-strand RNA, an essential mechanism in viral replication. BTN10 and BTN11 are two compounds that are under early-stage investigation as helicase inhibitors.
Internal ribosome entry site (IRES) inhibitors-
The IRES of HCV serves as a direct regulator for assembly of translation inititation complexes on viral mRNA. RNA molecules designed to interfere with the formation of a translationally active IRES complexes would ultimately inhibit particle formation. The front runners of this development field are HH363-50, HH363-10 and HH363. As in the case of entry inhibitor development, these compounds are still in preclinical trials.
However, HCV has high heterogeneity and high mutation rates, so it is likely that drug resistance will emerge during treatment, especially treatments with specific inhibtors of viral proteases and polymerases in a combination setting (Schinazi et al. 2010).
Projected pharmaceutical sales for 2011
Telaprevir has not yet been approved by the U.S. Food and Drug Administration, but if its "New Drug Application" (to be submitted in the fourth quarter of 2010) is approved, there will be significant economic implications.
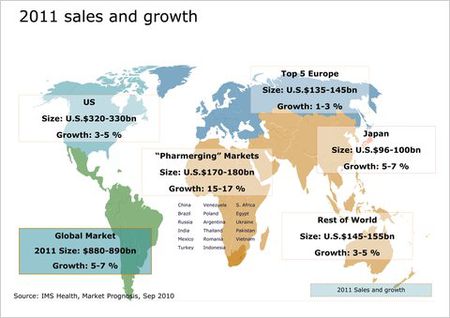
The current cost of 48 weeks of standard therapy is approximately US $35,000. Introduction of new anti-HCV drugs, like telaprevir, will likely be used in combination with the interferon/ribavirin treatment; this could double or even triple the full cost of treatment (Melnikova 2008). It is forecast that the global drug market will grow 5 to 7 percent in 2011. Telaprevir is one of five projected "blockbuster drugs" (drugs that are expected to have more than $1 billion in sales) for 2011. Despite the high cost of treating HCV, there is an upside to an expanding pharmaceutical market. "Pharmerging" Markets (emerging pharmaceutical markets) could also experience growth. (Figure 10; Wilson 2010). With a GDP of more than $8 trillion, China, a pharmerging market, is ready to become the world’s third-largest pharmaceutical market next year. The second tier of pharmerging markets includes: Brazil, Russia and India; each is expected to add $5-15 billion per country in annual sales to the global pharmaceutical market by 2013. A third tier of 13 countries is expected to contribute $1-5 billion per country in annual sales growth by 2013. It includes: Venezuela, Poland, Argentina, Turkey, Mexico, Vietnam, South Africa, Thailand, Indonesia, Romania, Egypt, Pakistan and Ukraine (IMS Health Incorporated 2010). Murray Aitken, IMS senior vice president in charge of research, says that the growth is, “driven by the level of economic development in these markets and the growing demand for access to medicines and a growing commitment by governments to help make those treatment options available to their citizens” (Wilson 2010). If Aitken’s assertion has any merit, there may be promise in drug treatment for HCV-infected individuals in both developed and developing countries, provided telaprevir is affordable and accessible.
References
Hunt, Margaret. 2006. Interferon. (27 Oct. 2010).
Vertex. 2010. Telaprevir (VX-950) (HCV Infection) Vertex Pharmaceuticals. (13 Oct 2010).
Moradpour, Darius et al. 2007. Replication of hepatitis C virus. Nature. 5:453-463.
Melnikova, Irena. 2008. Hepatitis C therapies. Nature. 7:779-800.
Clercq, Erik De. 2007. The design of drugs for HIV and HCV. Nature. 6: 1001-1017.
Wilson, Duff. 2010. Forecast sees drug-sales growth overseas. The New York Times. (13 Oct, 2010).
IMS Health Incorporated. 2010. Succeeding in pharmerging markets. (29 Oct. 2010).
Author
Sally Wilson, student of Joan Slonczewski for BIOL 238 Microbiology, 2010, Kenyon College.
