The Development of the Gut Microbiome in Young Children
Introduction to the Gut Microbiome
The gut microbiome is composed of trillions of microbes that form a protective barrier in the digestive system of humans as well as many other organisms. The gut microbiome, also known as gut flora or gut microbiota, is a part of the innate immune system that is essential for human health. The gut microbiome protects humans from pathogenic microbes and helps regulate the uptake of several important vitamins and other nutrients. Within this microbiome, there is an immense level of diversity of species. The majority of these species are bacteria, but various fungi and archaea have also been identified. [1]
Because the gut microbiota is such an important mechanism of defense in humans, it begins developing as soon as a baby is born. While the majority of bacterial infiltration into the gut occurs just after birth, an initial exposure to beneficial microbes takes place in the placenta in utero. [2] It has been found that the gut flora of infants develops for about 3 years to reach maturity. [1][3][4][5][6]
There are a variety of different factors that can alter the composition of the gut microbiome, including age, geography, diet, weight, and genetics. In children, maternal genes and health as well as diet and environment play a large role in the development and change of the microbiota (Figure 1).[1] Research has been done to show the effects of cesarean section, natural birth, breast feeding, and formula feeding on the neonatal microbiome. A healthy gut microbiome makes important contributions to human health and the regulation of metabolism. [1]
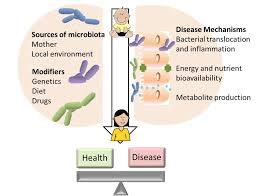
What Factors Influence the Composition of the Gut Microbiome?
Diet has been found to be one of the largest influencers of the composition of the gut microbiome. Not only does food provide the nutrients necessary to support a robust microbiome, but the microbes that are contained in the food also contribute to the diversity of the microbiome. An unhealthy diet often degrades the microbial diversity of the gut, which leaves the affected person more susceptible to inflammation and infection.[1][7][6] Diverse diets require the expression of different genes because some foods must be broken down in unique ways. Unhealthy and unbalanced diets, such as ones that are high in sugar and fats, processed foods, or are lacking fibers and important vitamins negatively affect the microbiome because of a lack of viable nutrients to sustain the gut flora. This can lead to an increased risk of illness, diabetes, and inflammatory bowel disease (IBD).[6] Malnourishment also results in a lack of proper nutrients to sustain a healthy and balanced microbial community in the gut.[7] Because diet is so influential in the configuration of the gut microbiome, any factors that affect diet come into play as well when discussing gut microbiota. Geography influences the genetics of a person and their access to different types of nutrients, which in turn alter the diversity of the microbiome. The regulation and expression of certain genes can lead to a more suitable environment for colonizing bacteria or can allow for the efficient breakdown of different compounds, which improves the conditions for the gut flora. Conversely, the suppression of a gene has the potential to create a disruptive environment for the gut flora.[6] These factors that can alter the composition of the gut microbiome in adults also influence the contents of breast milk in women.[8]
Prebiotics support and induce the growth of beneficial microbes, and allow the bacteria to thrive and diversify by providing nutrients. Probiotics, which are often found in fermented foods, are the helpful bacteria that promote a robust gut microbiome. Both prebiotics and probiotics are necessary for establishing and maintaining a healthy gut microbiome. [1]
The Gut Microbiome in Infants
The gut microbiome performs several necessary functions that sustain and enrich human life. The microbes that inhabit the gastrointestinal tract aide in the absorbance and utilization of nutrients. While children are not born with an inherently mature gut microbiome, the system develops over time as their lifestyle changes.[5]
Immediately after birth, babies experience an influx of bacterial exposure, both from their mother and their surroundings. [1][3][9][5][2][10] As children grow up and develop, their gut microbiome does as well because it is colonized by more diverse microbes. It is widely accepted that the gut microbiome of young children matures towards the composition of the microbiome of adults within the first three years of life.[1][3][4][6] While adults with similar lifestyles and geographical areas are more uniform in the composition of their gut microbiome, children have increased levels of variability between them.[6]
In the gut microbiomes of infants that are 1 to 10 days old, the bacteria Enterococcus and Streptococcus are the most prevalent.[4] After this initial period, the microbiome of children, especially those who are breastfed, is dominated by Bifidobacterium and Lactobacillus.[4][10][11] After the first year of life, the microbiome of children resembles the microbiome of the mother and is more functional and complicated. During breastfeeding, when milk is the main component of a child’s diet, lactose metabolism produces energy. As infants are weaned off of breast milk, which usually occurs after the first year in mothers who breastfeed, the gut flora develops more diverse metabolic pathways. These include the breakdown of carbohydrates, complex sugars, and starches, as well as fermentation and the synthesis of various nutrients and vitamins. [11]
While the microbiome of an infant is developing, it is particularly susceptible to the effects of antibiotics. Several species of bacteria contain or develop antibiotic resistance genes naturally. However, when antibiotics are introduced to the microbiome, the gut flora becomes more susceptible to invasion by pathogens, inflammation, and infection. Antibiotics also lower the diversity of the gut microbiome of children.[1]
Cesarean Section and Natural Birth
Vaginal Birth
Vaginal birth provides a newborn baby with an initial exposure to the flora of the mother, which in turn helps the gut microbiome develop and diversify. This initial “vaccination” from the mother’s vaginal flora sets the foundation for a healthy gut microbiome.[3][9][5] Vaginal delivery allows for the microbes of the mother as well as the bacteria of the surroundings to enter the gut of the newborn child. Because the first substantial exposure to microbes comes from the mother’s bacteria, the child’s microbiome resembles the mother’s for around 6 months to a year, until the baby’s own microbiome begins to fully develop and mature (Figure 2).[10][3][9][5][11]
The placenta has a concentration of bacteria of the Escherichia genus, which does not differ for vaginal birth and C-section, but may differ slightly between women. There are significant differences in the bacteria that inhabit the microbiome in babies born vaginally versus those delivered by C-section. For children born by vaginal delivery, Bifidobacterium, Parabacteroides, and some Bacteroides are present in the gut. Comparatively, the microbiome of a C-section baby includes Enterobacter, Haemophilus, Streptococcus, and other skin and oral bacteria. Vaginal birth leads to a slightly more developed microbiome that has loose resemblance to that of an adult.[11]
Most of the initial bacterial colonizers of the microbiome, which are mostly from the mother, decrease in number overtime. Certain maternal strains of bacteria influence the long term health of the child and his or her microbiome. Because the mother’s microbes are the first to colonize the child’s GI tract, they are more likely to adapt and successfully establish themselves in the child’s gut microbiome than the bacteria from other sources (Figure 2).[10] Selective pressures and environmental selection result in the maturation of the child’s microbiome as they grow older.[5]
Cesarean Section
A cesarean section, also known as a C-section or Cesarean Delivery (CD) is a surgical procedure in which the abdomen of a pregnant woman is cut open to remove the baby. This procedure can be done in emergency situations, or it can be voluntary. Because the child does not move through the birth canal, there is no initial exposure to the vaginal flora of the mother and the child’s gut microbiota do not closely resemble that of the mother. Instead, there is an increased proportion of bacteria from the surroundings, including the others present in the room and the room itself.[5][1][9][11] Often, a mother’s microbes are able to adapt to the system of the child better than other microbes, but the lack of vertical transfer of bacteria from the mother to the child means there is increased range and diversity of bacteria from the surroundings that initially colonizes the intestines.[5] However, there is evidence of a delay in the establishment of the gut flora in babies delivered by C-section. This delay can last up to six months, which endangers the balance and establishment of healthy gut microbiota.[1][9][11]
Although the diversity of bacteria in the gut of children born by C-section is initially higher, the delay in colonization leads to an eventual decrease in diversity, which weakens the microbiome and its response to pathogens. [1] While some species of bacteria are able to colonize the gut microbiome of a child delivered by C-section quickly, they often are not able to adapt to the environment or establish themselves in a sustainable manner. This hindrance to colonization can leave these children more vulnerable to infection, inflammation, or other diseases usually prevented by a healthy gut. It has been hypothesized that the anesthesia and other drugs given to a mother during a C-section may have an effect on the bacterial colonization rates in infants, but more research still needs to be done to provide evidence for this idea.[9]
Because C-sections do not involve the hormones and chemical signals that are necessary for the induction of labor and vaginal birth, the procedure can also affect the production or composition of breast milk. The changes in milk production due to the lack of hormonal signals can include a decrease of human milk oligosaccharides (HMOs), which are beneficial to the human gut.[8]
The genetics of obesity are often inherited by children, leading to an increased risk of this condition in childhood. Mothers who are obese or overweight often have C-sections, particularly emergency ones. Because of the disturbances a C-section has on a balanced and developed gut microbiome in infants, the likelihood of a child becoming overweight in their first few years of life is increased. The combination of these two factors—obesity genetics and changes in microbial composition in the gut—lead to increased rates of childhood obesity in the children of overweight mothers who underwent emergency C-sections.[12]
Breast Milk and Formula Milk
Components of Breast Milk
Breastfeeding has an influential role in the shaping of a healthy gut microbiome, which leads to reduced instances of gastrointestinal (GI) inflammation, obesity, and respiratory ailments or allergies both in infancy and throughout life. After a newborn child’s initial exposure to various microbes in their surroundings, the composition of the gut microbiome is relatively diverse; however, few species have successfully adapted to the conditions. Breast milk, which is full of diverse bacterial species, prebiotics, probiotics, and nutrients from the mother, provides an initial defense against potential pathogens and increases the productivity and effectiveness of the gut microbiome while development occurs.[8] Breastfeeding exposes infants to additional bacteria on the mother’s skin, which in turn increases the diversity of the gut flora.[1]
Breast milk contains metabolites which have a large impact on which microbes adapt and thrive in the gut, because of their ability to foster new and beneficial microbes. Some of the most numerous metabolites found in breast milk include lactose, lipids, low density lipoproteins (LDL), very-low density lipoproteins (VLDL), human milk oligosaccharides (HMOs) and amino acids.[8] These compounds, which are necessary for the varied metabolic needs of different species, are accompanied by diverse bacteria, including Bifidobacterium, Bacteroides, Lactobacillus, Strepococcus, and Enterococcus (Figure 3).[4] Bifidobacterium are crucial in establishing and maintaining a healthy gut microbiome in infants because they produce acetate, which helps provide an extra defense against infection.[8][4][10][6]
Nitrogen is a limiting nutrient in most environments, including the gut microbiome. Breast milk contains urea, which can be broken down into ammonia, a nitrogen rich compound. Because nitrogen is crucial for amino acid production, these nitrogen rich compounds sustain and enrich the microbes that reside in the gut microbiome.[8][6]
Human milk oligosaccharides (HMOs) are prebiotic compounds that lay a foundation for healthy colonization of the gut microbiome. As a metabolite, HMOs promote the growth of beneficial bacteria in the gut. HMOs also play a role in the immune system by activating pathogen resistant mechanisms and reducing inflammatory cytokines. In addition, HMOs deter pathogens from entering into and residing in the epithelial walls of the intestines.[8]
The gut microbiome is often referred to as an extension of the immune system, because the two work together to maintain a healthy balanced GI tract. Breast milk transports secretory immunoglobulin A (SIgA) or secretory antibodies, which promote protection from pathogens in the intestines, to the gut. SIgA contributes to the growth of mutualistic microbes, provides broad immune protection against antigens, and regulates gene expression in the intestinal epithelia. The SIgA in breast milk regulates the expression of genes that maintain internal homeostasis by reducing threats of infection and inflammation, increasing the productivity of gut flora, and reducing the likelihood of developing inflammatory bowel disease (IBD).[3] In addition, the SIgA sends signals to the epithelial cells that aide them in the establishment of a barrier to pathogens. A lack of SIgA from breast milk leads to the expression of genes resulting in IBD and other GI issues, as well as a lower rate of functionality in the gut microbiome.[3]
Formula Milk
Formula is a manufactured version of breast milk that aims to achieve similar results in nourishing an infant for approximately a year after birth. In recent years, there has been an emphasis on making formula more similar to breast milk, which contains nutrients and products that are essential for the health of the microbiome. Prebiotics and probiotics are two of the most important components of breast milk that help establish a healthy gut. The importance of these compounds has become apparent and they are widely added to formula. Although formula is similar to breast milk, the benefits of microbial exposure from the mother are not available.[1][3][4]
The most apparent difference between breast milk and formula milk is the types and numbers of bacteria that are present. Breast milk contains large proportions of Lactobacillus and Bifidobacterium. This composition leads to the domination of these bacteria in the gut of breast-fed infants. In contrast, formula fed infants have a much more diverse range of bacterial inhabitants in their gut microbiomes.[1] While diversity is important in the gut microbiome, the establishment of Bifidobacterium has been linked to a reduction in inflammation and other ailments of the GI tract, and Lactobacillus aids in lactose digestion. Due to a reduction in colonization levels of these bacteria, formula-fed babies tend to see higher rates of infection, inflammation, and IBD than infants who are breastfed.[4]
Conclusion
The gut microbiome is a rich and diverse region of the body that plays a large role in the health and wellness of humans throughout their lives. It can be influenced by a number of things including geography, age, diet, nutrient availability, and genetics, but remains stable when it is well taken care of. After maturation and refinement of different metabolic activities, the gut microbiome provides an internal barrier to harmful pathogens or other ailments. Vaginal birth and breast milk add level of nutrients, bacteria, and antibodies that are inherited from the mother, and C-section delivery and formula milk lead to a more diverse but less stable community of microbes in the infant gut. The successful and balanced development of the gut microbiome is an essential piece of human health.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 Mohammadkhah AI, Simpson EB, Patterson SG, Ferguson JF. Development of the gut microbiome in children, and lifetime implications for obesity and cardiometabolic disease. Children. 2018;5(12):160.
- ↑ 2.0 2.1 Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65-237ra65.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Rogier EW, Frantz AL, Bruno MEC, Wedlund L, Cohen DA, Stromberg AJ, et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci. 2014;111(8):3074–9.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Solís G, de Los Reyes-Gavilan CG, Fernández N, Margolles A, Gueimonde M. Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe. 2010;16(3):307–10.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 2018;24(1):133–45.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010;107(33):14691–6.
- ↑ 7.0 7.1 David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 Gómez-Gallego C, Morales JM, Monleón D, Du Toit E, Kumar H, Linderborg KM, et al. Human breast milk NMR metabolomic profile across specific geographical locations and its association with the milk microbiota. Nutrients. 2018;10(10):1355.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Grölund M-M, Lehtonen O-P, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28(1):19–25.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690–703.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7.
- ↑ Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB, et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr. 2018;172(4):368–77.
Edited by Sophia Knaysi, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2020, Kenyon College.