The Gut-Brain Axis: The Human Gut Microbiome and Anxiety and Depression
Introduction
By Samantha Lee
Humans have evolved throughout the many years with microbes. Microbes play an important role in not only human health but also human disease. The human intestinal tract contains a wide variety of microorganisms, all of which have a large impact on health and disease [2]. This microbiota has recently been studied more in depth as researchers discovered that microbes have a larger impact on human health than once understood. Antibiotics and diet have been shown to alter the diversity of the gut microbiota, leading to the possibility of irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) since patients with IBS and IBD have been shown to have unstable gut microbial populations [3].
The human gut contains a large number of microbes that live in the gastrointestinal tract of humans and other animals [4]. The gastrointestinal tract is colonized by this community of microbes after birth, and these microbes play an important role in digestion, metabolism, and immune function [3]. They can ferment food and supply nutrients and energy to a host and to its immune systems [5]. The intestinal microbiota has a wide diversity of microbial species, with around 40,000 bacterial species that are influenced by vertical transmission from one’s mother, genetic makeup, diet, medications, gastrointestinal infections, and stress [3].
The human gut microbiota can be grouped by three major bacteria enterotypes: Bacteroides, Prevotella, and Ruminococcus [6]. Bacteroides are Gram-negative, obligate anaerobic bacteria. They make up a significant portion of the human fecal bacterial population [7]. Prevotella is another Gram-negative bacteria, and it is thought to have a common ancestor with Bacteroides [8]. It is more common in the gut of individuals who eat primarily a diet filled with carbohydrates, since it is able to break down fibers and plant glycans [8][9]. Ruminococcus, on the other hand, is an anaerobic, Gram-positive bacteria. Certain species of Ruminococcus have been found to be less prevalent in individuals with IBD [10]. Treatment with antibiotics, in addition to changes in diet, environment, etc., can affect the intestinal gut microbiota and can cause lifelong changes to the makeup of this community, including these three major bacterial enterotypes.
Neurotransmitter Release and Signaling
Neurotransmitters are important signaling molecules secreted by neurons that send signals to other cells. Each type of neurotransmitter is produced in a different way. Dopamine is a neurotransmitter involved in reward, and it is a precursor molecule for norepinephrine and epinephrine [11]. It is synthesized by the removal of a carboxyl group from L-DOPA. Dopamine plays a large role in the reward aspect found in addictive drugs by blocking dopamine reuptake or increasing dopamine release [12]. Additionally, dopamine is involved in motor control and the release of various hormones.
Norepinephrine, which is produced by the conversion of dopamine, is known for arousal and alertness, but may also have a role in memory, learning, and attention [14]. Due to its arousal properties, norepinephrine can increase heart rate and blood pressure, can release glucose from storage, can increase blood flow, and can cause restlessness and anxiety. Norepinephrine is an important neurotransmitter in the development and progression of mood disorders, in particular anxiety.
Serotonin is a neurotransmitter largely involved in the development and progression of mood disorders. Serotonin is secreted by enterochromaffin cells, which are located along the epithelium lining of the digestive tract [15]. It is involved in mood, cognition, learning, and memory, as well as many physiological functions [16]. Around 95% of serotonin in the body is located in the gastrointestinal tract [17].
In order to transmit signals, neurons release neurotransmitters into the presynaptic terminal. These neurotransmitters are only released following an action potential, where a rapid depolarization of the neuron occurs. These released neurotransmitters bind specific receptors located on the next neuron. These receptors are specific to the neurotransmitter. Depending on the neurotransmitter released, the signal sent to the following neuron could be excitatory or inhibitory. Following this binding, neurotransmitters are removed from the synaptic cleft, or the space between the two neurons [18]. They can be removed through diffusion, where the neurotransmitters slowly drift out of the synaptic cleft, through enzyme degradation, or through reuptake, where they are reabsorbed into the presynaptic neuron.
Mood Disorders
Mood disorders are mental health conditions that affect one’s emotions and behaviors [19]. They include depression disorders, anxiety disorders, and manic disorders. The development of mood disorders is not fully understood, but genetics and environmental factors seem to play a role in the progression of these forms of disorders [20]. Additionally, there is evidence suggesting one’s neuroticism is a predictor for the development of a mood disorder [21].
There are differences in prevalence of mood disorders based on sex. For example, in the United States, women are more likely to be diagnosed with a stress-related mood disorder than men [22]. All diagnoses of mood disorders come from the DSM-5, the standard manual of mental disorders with the diagnostic criteria and treatment options for every form of mental disorder known. It was published by the American Psychiatric Association in 2013, and a revised version of the DSM-5 was published in 2022.
Depression
Major depressive disorder is one of the most common mood disorders. It can be present in 121 million people at once [23] and symptoms include low mood or anhedonia and changes to physical and physiological functions [20]. Many people with depression have episodes beginning during adolescence, and these episodes are recurrent [20][24].
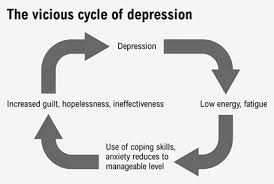
Depression can develop for a multitude of reasons. “Susceptibility genes, neurochemical imbalances, brain circuitry dysfunction, faulty information processing, negative cognitions, social or environmental sources of vulnerability, and precipitating stressors” [20] are all potential reasons for the development and progression of depression.
One of the key causes of depression is through genetics. Genetic influences are similarities of depression measures that increase with shared genes [20]. Families are used to study this cause of depression by comparing parents with depression and the number of their offspring with depression [20]. Twin studies compare behavior between identical and fraternal twins. Since both twins have the same family or possible common environment, this form of study shows the overall impact of genes and is the strongest piece of evidence for the heritability of depression [20].
In major depressive disorder, the most common genetic markers are involved in serotonergic neurotransmission [20]. Abnormalities in serotonin have been linked with the pathophysiology of depression [20].
Anxiety
While day to day anxiety is common, anxiety disorders are another common mood disorder, characterized by uncontrollable feelings of anxiety and fear that impair one’s social, occupational, and personal activities [26]. It affects about 16.4% of the population or around 40 million people in the United States [27]. Symptoms of anxiety include fatigue, restlessness, difficulty concentrating, increased heart rate, and chest pain [26]. There are many types of anxiety disorders, including generalized anxiety disorder, social anxiety disorder, obsessive-compulsive disorder, panic disorder, and post-traumatic stress disorder.
Norepinephrine and serotonin are two important neurotransmitters for anxiety disorders. Norepinephrine is involved in the pathogenesis and regulation of anxiety disorders. Serotonin, in addition to its involvement in major depression disorder, is involved in the pathophysiology of OCD and other anxiety disorders [28].
Treatments
Treatment options for both anxiety and depression disorders are similar, including various therapies, medications, and lifestyle changes. The first line of treatment is typically some form of therapy, whether it be cognitive behavioral therapy (CBT) or more specific forms, like exposure and response prevention (ERP). CBT is focused on changing cognitions, such as thoughts, beliefs, and attitudes, and behaviors associated with those cognitions while also developing coping strategies [30].
Due to the involvement of serotonin, selective serotonin reuptake inhibitors, or SSRIs, are one of the common drugs used for the treatment of depression and anxiety disorders. These drugs work by blocking the reuptake molecules responsible for bringing serotonin molecules back into the neuron [31]. This allows serotonin to stay outside of the neuron for longer periods of time and have more effects on the brain.
SNRIs are another common form of medication. These are selective norepinephrine reuptake inhibitors and have a similar mechanism of action as SSRIs [32]. While there are a variety of treatments available for both depression and anxiety, there are forms of mood disorders that are treatment resistant, meaning they do not respond to any form of treatment available.
The Gut-Brain Axis
The gut-brain axis is composed of the brain, the spinal cord, the autonomic nervous system (sympathetic, parasympathetic, and enteric), and the HPA axis [33]. First, the enteric nervous system (ENS) is a portion of the autonomic nervous system that produces over 30 neurotransmitters and has more neurons than the spine, with around 100 to 500 million neurons [34]. It is made up of plexes, which are networks of interlacing neurons. The submucosal plexus of the enteric nervous system regulates gastrointestinal blood flow and controls epithelial cell functions and secretions [35]. The myenteric plexus regulates the relaxation and constriction of the intestinal wall [35], or peristalsis. Serotonin plays a large role in peristalsis by binding to serotonin receptors on neurons, and ultimately activating those neurons, involved in initiating relaxation and constriction [36]. Using GABA, dopamine, norepinephrine, serotonin, and other neurotransmitters, the gut bacteria can interact with intestinal cells and enteric nervous system and with neuroendocrine and metabolic systems while also communicating with the central nervous system, which is comprised of the spine and brain, through the enteric nervous system [37]. These signals are transported to the central nervous system through the afferent vagus nerve fibers [37].
The vagus nerve is the tenth cranial nerve and plays a large role in the relationship between the gut, the brain, and inflammation [34]. It travels from the brainstem, through the neck and thorax, and down to the abdomen [34]. The vagus nerve is responsible for regulating internal organ function [39]. The most important function of the vagus nerve is to bring information from the inner organs to the brain [34]. It is one portion of the gut-brain axis.
The vagus nerve afferent pathways regulate hypothalamus-pituitary-adrenal (HPA) axis activity [40]. The HPA axis is the primary pathway activated during a stressful situation. The HPA axis is the primary pathway activated during a stressful situation. It is composed of the hypothalamus, a brain structure involved in regulating body temperature and hunger, the pituitary gland, which produces hormones to control growth, metabolism, and reproduction, and the adrenal gland, which synthesizes hormones to regulate immune function and responses to stress. Cortisol is produced by the adrenal gland and is the hormone released that is associated with stress. The production of cortisol leads to the availability of glucose and the suppression of immune responses [41].
There have been many studies looking at the relationship between the gut and mental health. One study, analyzing the correlation between diet and depression, showed that depression in females was associated with increased fermentation of carbohydrates [42]. This fermentation indirectly implicates changes in the gut microbiota composition, since more fermentation means more bacteria needed for these processes.
When looking at specific bacteria that have possible influences on mental health, a variety of bacteria have some effects on mental health. Mice have been shown to have altered, anxiety-like behavior when infected with Campylobacter jejuni [43], triggering activity of vagal nerve ascending pathways [44]. These pathways include the nucleus tractus solitarius and lateral parabrachial nucleus [44]. Additionally, chronic Helicobacter pylori infection changes gastric physiology, leading to delayed gastric emptying and visceral sensitivity, and abnormal feeding behavior [45], accompanied by the down-regulation of Proopio-melanocortin (POMC), a regulatory peptide, and the upregulation of TNF-ɑ [3].
Bifidobacterium and Anxiety and Depression
With chronic infection from a non-invasive parasite and chemically-induced colitis, there was an increase in anxiety and depression-like behavior in mice [46]. However, these symptoms were normalized by treatment with probiotic Bifidobacterium longum NC3001 [3]. B. longum is a Gram-positive bacterium that is sometimes added to food products [47]and can produce lactic acid [48]. B. longum was not found to improve gut inflammation, but there was a reduction in anxiety behavior in the mice with this treatment[3]. Neurons treated with B. longum fired fewer action potentials when reacting to a depolarizing current [46].
Desbonnet et al. (2008) looked at the potentially antidepressant effects of Bifidobacterium infantis on rats [49]. Following treatment with B. infantis, rats underwent forced-swim tests, while also being tested for immune, neuroendocrine, and monoaminergic activity [49]. There was no change in swim behaviors in rats treated with B. infantis, but concentrations of serotonin and dopamine metabolites in the frontal and amygdala cortices increased, along with a decrease in pro-inflammatory cytokines [49]. Serotonin and dopamine, as well as pro-inflammatory cytokines, have potential effects on the development of depression, indicating that B. infantis may have antidepressant properties.
Lactobacillus and Anxiety and Depression
Lactobacillus rhamnosus is a Gram-positive bacterium that can be found in the female urogenital tract [51]. It is a common bacterium in daily probiotics, used as a supplement to improve gut health. In relation to the gut-brain axis, L. rhamnosus has been found to have an impact on GABA, an inhibitory neurotransmitter that decreases activity in the following neuron. L. rhamnosus treatment in mice promoted exploratory behavior and decreased depression-like behavior, along with alterations in the brain of mRNA for GABA(B1b) and GABA(Aɑ2), both of which are receptors for GABA molecules [50]. The change in GABA(B1b) and GABA(Aɑ2) mRNA being associated with decreased depression-like behavior indicates that GABA has possible effects on the development of depression. L. rhamnosus has altered the expression of GABA receptors in the brain, leading to a decrease in anxiety-like symptoms as well [52]. This aligns with the previous piece of evidence to indicate the relationship between GABA with both anxiety and depression disorders. When looking at a specific type of anxiety, Obsessive-Compulsive Disorder (OCD), mice treated with L. rhamnosus had a decrease in OCD-like symptoms, and these findings were similar in humans when treated with L. helveticus [53][54].
HPA Axis and Anxiety
The HPA axis is active in acute anxiety situations, due to cortisol reducing the perception of fear by hindering memory retrieval [55]. However, in chronic anxiety, a disconnect develops between the stressor and the behavioral consequence, allowing individuals to function normally while still having anxiety-like feelings [56]. The HPA axis ends up being chronically active in these cases of persistent anxiety and can cause harm by decreasing hippocampal serotonin receptors from the high amount of corticoids released during HPA activation [57]. These effects then lead to further impairment of coping mechanisms, thus prolonging the cycle and leading to more long-term damage.
In rodents, maternal separation and social isolation are common behavioral stressors used to understand the effects of long-term stress on the brain. When looking at early maternal separation, rodents showed changes in the brain circuitry involved in stress reactivity, specifically with an exaggeration of HPA axis activity [58]. When looking at social isolation, rodents showed abnormalities in behavior that reflects that of human depression and anxiety disorders [59]. While animal models are not fully accurate, they provide significant results that can further the understanding of human behavior.
Patients suffering from anxiety have been shown to have chronic stress-induced adrenal hyperactivity and elevated cortisol levels, indicating an overactive HPA axis [60]. Long-term exposure to elevated cortisol levels can cause damage to hippocampal receptors and hippocampal neurons [61], which would lead to higher cortisol concentrations, furthering the damage to the hippocampus.
Changes in the HPA axis may be due to the exposure to stressful situations during critical development stages. For example, rates of childhood abuse have been associated with patients suffering from anxiety disorders [60]. These stressors during important developmental stages can cause long-term changes in the HPA axis during these critical periods, leading to chronically low basal cortisol levels and impacting mood and mood disorders during adulthood [62].
Other Evidence of the Gut-Brain Axis
Evidence connecting the gut microbiome to the development of anxiety and depression suggests that overall diversity in the gut microbiome has associations with the presence of anxiety and depression-like symptoms. When analyzing stool sample microbial diversity in individuals suffering from depression, those suffering from depression had less diversity in stool samples than normal individuals [52]. This suggests that lower diversity of microbes in the gut can lead to the development of depression. Similarly, in patients suffering from Generalized Anxiety Disorder, stool samples showed lower diversity in the microbial community [63].
Irritable Bowel Syndrome (IBS) and Irritable Bowel Disease (IBD) have been shown to have instability in gut microbiome populations [3]. This instability can indicate lower diversity in the gut, which has been associated with depression and anxiety. When looking at potential comorbidities for IBS patients, around 50% of patients had a comorbidity with depression or anxiety disorders [64]. The interaction between the gut microbiome and mood disorders is an important area for research. Understanding this relationship more can lead to better treatment plans for mood disorders.
Current Research on Vagus Nerve Stimulation
Vagus nerve stimulation (VNS) is a treatment for epilepsy that has been used since the 1990s [28]. Sensory afferent nerve fibers stimulated from VNS will send signals to the brain and terminate in the nucleus solitary tract (NTS), a major nucleus in the brain that receives and responds to sensory signals [28]. These nerve fibers are the main way to send information to the brain from organs within the gut [28]. VNS has been shown to increase turnover of serotonin within the brain and also to increase activity in the thalamus, insula, amygdala, and brain stem [66][67]. All of these brain regions are involved in anxiety modulation or perception [67]. VNS is now being used as treatment for patients with chronic treatment-resistant depression [28].
Success in decreasing depression symptoms from VNS may be due to the inhibition of the production of proinflammatory cytokines [68]. These cytokines upregulate the release of inflammatory molecules, and chronic exposure to inflammatory signals can lead to the development of depression [69]. Additionally, some studies of VNS success on depression symptoms showed significant anti anxiety effects in addition to improvements on depression [70]. However, VNS treatment was only well tolerated in patients suffering from anxiety with limited improvement in symptoms [28]. This minimal improvement in symptoms may be due to the increase in serotonin turnover in the brain or to the increase in activity in brain regions involved in anxiety modulation.
Conclusion
Mood disorders, specifically anxiety and depression disorders, are a very common problem in the United States. In 2011, mood disorders were the leading cause of hospitalization in children between the ages of 1 and 17, while also accounting for the top diagnosis for Medicaid patients with recurring healthcare visits in 2012 [71][72]. While future research is necessary to illuminate further mechanisms of the connection between mental illness and the gut microbiome, the current research on the gut-brain axis provides some hope that there could be a more in depth understanding of mental illness, in addition to some possible new treatment options for those suffering. Comprehension of this complex, yet influential, pathway can help patients suffering from debilitating illnesses.
References
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024
- ↑ Gut Microbiome. https://www.sciencedirect.com/topics/medicine-and-dentistry/gut-microbiome
- ↑ Eckburg, P.B., Bik, E.M, Bernstein, C.N., et al. (2005) Diversity of the Human Intestinal Microbial Flora. Science Express. 308(5728): 1635-1638. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1395357/
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Bercik, P., Collins, S.M, and Verdo, E.F. (2012) Microbes and the gut-brain axis. Neurogastroenterology and Motility. 24(5):405-413. Available at: https://pubmed.ncbi.nlm.nih.gov/22404222/
- ↑ Moszak, M., Szulińska, M., and Bogdański, P. (2020) You Are What You Eat – The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients. 12(4):1096. Available at: https://pubmed.ncbi.nlm.nih.gov/32326604/
- ↑ Lee, Y. and Kim, Y. (2021) Understanding the Connection Between the Gut-Brain Axis and Stress/Anxiety Disorders. Current Psychiatry Reports. 23(5):22. Available at: https://pubmed.ncbi.nlm.nih.gov/33712947/
- ↑ Arumugam, M., Raes, J., Pelletier, E., et al. (2011) Enterotypes of the human gut microbiome. Nature. 473: 174–180. https://www.nature.com/articles/nature09944
- ↑ Madigan, M., Martinko J. (2005). Brock Biology of Microorganisms. 11th ed. Prentice Hall.
- ↑ 8.0 8.1 Ley, R.E. (2016) Gut microbiota in 2015: Prevotella in the gut: choose carefully. Nature Reviews. Gastroenterology & Hepatology. 13(2):69–70. https://pubmed.ncbi.nlm.nih.gov/26828918/
- ↑ Wu, G.D., Chen, J., Hoffmann, C., et al. (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science. 334(6052):105–108. https://pubmed.ncbi.nlm.nih.gov/21885731/
- ↑ Nagao-Kitamoto, H. and Kamada, N. (2017). Host-microbial Cross-talk in Inflammatory Bowel Disease. Immune Network. 17(1):1–12. https://pubmed.ncbi.nlm.nih.gov/28261015/
- ↑ Musacchio, J.M. (2013) Biochemistry of Biogenic Amines. Springer.
- ↑ Wise, R.A. and Robble, M.A. (2020). Dopamine and Addiction. Annual Review of Psychology. 71(1):79–106. https://pubmed.ncbi.nlm.nih.gov/31905114/
- ↑ Cleveland Clinic. (2022) Neurotransmitters [online]. Available from: https://my.clevelandclinic.org/health/articles/22513-neurotransmitters [accessed 14 April 2024].
- ↑ Borodovitsyna, O., Flamini, M., and Chandler, D., (2017) Noradrenergic Modulation of Cognition in Health and Disease. Neural Plasticity. https://pubmed.ncbi.nlm.nih.gov/28596922/
- ↑ Bertrand, P,P. and Bertrand, R.L. (2010) Serotonin release and uptake in the gastrointestinal tract. Autonomic Neuroscience. 153(1–2):47–57. https://pubmed.ncbi.nlm.nih.gov/19729349/
- ↑ Young, S.N. (2007) How to increase serotonin in the human brain without drugs. Journal of Psychiatry & Neuroscience. 32(6):394–399. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2077351/
- ↑ Gershon, M.D. and Tack, J. (2007) The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 132(1):397-414. https://pubmed.ncbi.nlm.nih.gov/17241888/
- ↑ Chergui, K., Suaud-Chagny, M.F., and Gonon, F. (1994). Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 62(3):641–645. https://pubmed.ncbi.nlm.nih.gov/7870295/
- ↑ Cleveland Clinic. (2022) Mood Disorders [online]. Available from: https://my.clevelandclinic.org/health/diseases/17843-mood-disorders [accessed 10 April 2024].
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 20.8 Lau, J.Y.F. and Eley, T.C. (2010) The Genetics of Mood Disorders. Annual Review of Clinical Psychology. 6:313-37. https://pubmed.ncbi.nlm.nih.gov/20001729/
- ↑ Jeronimus, B.F., Kotov, R., Riese, H., and Ormel, J. (2016) Neuroticism's prospective association with mental disorders: A meta-analysis on 59 longitudinal/prospective studies with 443 313 participants. Psychological Medicine. 46(14):2883–2906. https://pubmed.ncbi.nlm.nih.gov/27523506/
- ↑ Rosinger, Z. (2020) Sex-dependent effects of chronic variable stress on discrete corticotropin-releasing factor receptor 1 cell populations. Physiology & Behavior. 219:112847. https://pubmed.ncbi.nlm.nih.gov/32081812/
- ↑ Bebbington P. (2001) The World Health Report 2001. Social Psychiatry and Psychiatric Epidemiology. 36:473–74. https://pubmed.ncbi.nlm.nih.gov/11768843/
- ↑ Kim-Cohen, J., Caspi, A., Moffitt, T.E., et al. (2003) Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Archives of General Psychiatry 60:709–17. https://pubmed.ncbi.nlm.nih.gov/12860775/
- ↑ Department of Health: Government of Western Australia. Depression - reversing the vicious cycle [online]. Available from: https://www.healthywa.wa.gov.au/Articles/A_E/Depression-reversing-the-vicious-cycle [accessed 14 April 2024].
- ↑ 26.0 26.1 Diagnostic and statistical manual of mental disorders 5th edition: DSM-5. (2013) American Psychiatric Association.
- ↑ Kessler, R.C. (2007) The global burden of anxiety and mood disorders: putting the European Study of the Epidemiology of Mental Disorders (ESEMeD) findings into perspective. Journal of Clinical Psychiatry. 68:10-19. https://pubmed.ncbi.nlm.nih.gov/17288502/
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 George, M.S., Ward, H.E., Ninan, P.T., et al. (2008) A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimulation. 1:112-121. https://pubmed.ncbi.nlm.nih.gov/20633378/
- ↑ McGovern Medical School. (2019) What is CBT? [online]. Available from: https://med.uth.edu/psychiatry/2019/11/27/what-is-cbt/ [accessed 14 April 2024].
- ↑ Benjamin, C.L., Puleo, C.M., Settipani, C.A., et al. (2011) History of cognitive-behavioral therapy in youth. Child and Adolescent Psychiatric Clinics of North America. 20(2):179–189. https://pubmed.ncbi.nlm.nih.gov/21440849/
- ↑ Mayo Clinic. (2019) Selective serotonin reuptake inhibitors (SSRIs) [online]. Available from: https://www.mayoclinic.org/diseases-conditions/depression/in-depth/ssris/art-20044825 [accessed 10 April 2024].
- ↑ Stein, M.B. and Sareen, J. (2015) CLINICAL PRACTICE. Generalized Anxiety Disorder. The New England Journal of Medicine. 373(21):2059–2068. https://pubmed.ncbi.nlm.nih.gov/26580998/
- ↑ Carabotti, M., Scirocco, A., Maselli, M.A., and Severi, C. (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology. 28:203–209. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4367209/
- ↑ 34.0 34.1 34.2 34.3 Breit, S., Kupferberg, A., Rogler, G., and Hasler, G. (2018) Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Frontiers in Psychiatry. 9(44). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5859128/
- ↑ 35.0 35.1 Furness, J.B. (2016) Integrated neural and endocrine control of gastrointestinal function. Advances in Experimental Medicine and Biology. 891:159–173. https://pubmed.ncbi.nlm.nih.gov/27379644/
- ↑ Schemann, M. and Neunlist, M. (2004) The human enteric nervous system. Neurogastroenterology Motility. 16(1): 55-59. https://pubmed.ncbi.nlm.nih.gov/15066006/
- ↑ 37.0 37.1 Dicks, L.M.T. (2024) Our Mental Health is Determined by an Intrinsic Interplay between the Central Nervous System, Enteric Nervous System, and Gut Microbiome. International Journal of Molecular Sciences. 25(1):38. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10778721/
- ↑ Psych Scene Hub. (2023) The Simplified Guide to the Gut-Brain Axis: How The Gut and The Brain Talk to Each Other [online]. Available from: https://psychscenehub.com/psychinsights/the-simplified-guide-to-the-gut-brain-axis [accessed 14 April 2024].
- ↑ Babic, T. and Browning, K.N. (2014) The role of vagal neurocircuits in the regulation of nausea and vomiting. European Journal of Pharmacology. 722:38–47. https://pubmed.ncbi.nlm.nih.gov/24184670/
- ↑ Howland, R.H. (2014) Vagus nerve stimulation. Current Behavioral Neuroscience Reports. 1:64–73. https://pubmed.ncbi.nlm.nih.gov/24834378/
- ↑ del Rey, A., Chrousos, G. P., and Besedovsky, H. O. (2008). The Hypothalamus-Pituitary-Adrenal Axis. NeuroImmune Biology. 1 ed. Elsevier Science.
- ↑ Ledochowski, M., Sperner-Unterweger, B., and Fuchs, D. (1998) Lactose malabsorption is associated with early signs of mental depression in females: a preliminary report. Digestive Diseases and Sciences. 43: 2513–7. https://pubmed.ncbi.nlm.nih.gov/9824144/
- ↑ Lyte, M., Varcoe, J.J., and Bailey, M.T. (1998) Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiology and Behavior. 65: 63–8. https://pubmed.ncbi.nlm.nih.gov/9811366/
- ↑ 44.0 44.1 Gaykema, R.P., Goehler, L.E., and Lyte, M. (2004) Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain, Behavior, and Immunity. 18: 238– 45. https://pubmed.ncbi.nlm.nih.gov/15050651/
- ↑ Bercı´k, P., De Giorgio, R., Blennerhassett, P., et al. (2002) Immune-mediated neural dysfunction in a murine model of chronic Helicobacter pylori infection. Gastroenterology. 123: 1205–15. https://pubmed.ncbi.nlm.nih.gov/12360482/
- ↑ 46.0 46.1 Bercik P., Verdu E.F., Foster J.A. et al. (2010) Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 139: 2102–2112. https://pubmed.ncbi.nlm.nih.gov/20600016/
- ↑ Yazawa, K.,Fujimori, M., Amano, J., et al. (2000) Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Therapy. 7(2):269–274. https://pubmed.ncbi.nlm.nih.gov/10770636/
- ↑ Yuan, J., Zhu, L., Liu, X., et al. (2006) A Proteome Reference Map and Proteomic Analysis of Bifidobacterium longum NCC2705. Molecular & Cellular Proteomics. 5(6):1105–1118. https://pubmed.ncbi.nlm.nih.gov/16549425/
- ↑ 49.0 49.1 49.2 Desbonnet, L., Garrett, L., Clarke, G., et al. (2008) The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. Journal of Psychiatric Research. 43: 164–174. https://pubmed.ncbi.nlm.nih.gov/18456279/
- ↑ 50.0 50.1 Bravo, J.A., Forsythe, P., Chew, M.V., et al. (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences. 108: 16050–16055. https://pubmed.ncbi.nlm.nih.gov/21876150/
- ↑ de Vrese, M., Laue, C., Papazova, E., et al. (2019) Impact of oral administration of four Lactobacillus strains on Nugent score - systematic review and meta-analysis. Beneficial Microbes. 10(5):483–496. https://pubmed.ncbi.nlm.nih.gov/31012733/
- ↑ 52.0 52.1 Jiang, H., Ling, Z., Zhang, Y., et al. (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity. 48:186–194. https://pubmed.ncbi.nlm.nih.gov/25882912/
- ↑ Messaoudi, M., Violle, N., Bisson, J.-F., et al. (2011) Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2(4):256–261. https://pubmed.ncbi.nlm.nih.gov/21983070/
- ↑ Kantak, P.A., Bobrow, D.N., and Nyby, J.G. (2014) Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behavioral Pharmacology. 25(1):71–79. https://pubmed.ncbi.nlm.nih.gov/24257436/
- ↑ Soravia, L.M., Heinrichs, M., Aerni, A., et al. (2006) Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences. 103:5585- 90. https://pubmed.ncbi.nlm.nih.gov/16567641/
- ↑ Deakin, J.F.W. and Graeff, F.G.(1991) 5-HT and mechanisms of defense. Journal of Psychopharmacology. 5:305-315. https://pubmed.ncbi.nlm.nih.gov/22282829/
- ↑ Graeff, F.G., Garcia-Leal, C., Del-Ben, C.M., and Guimarães, F.S. (2005) Does the panic attack activate the hypothalamic-pituitary-adrenal axis? Anais da Academia Brasileira de Ciências. 77:477-491. https://pubmed.ncbi.nlm.nih.gov/16127553/
- ↑ Tyrka, A.R., Wier, L., Price, L.H., et al. (2008) Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biological Psychiatry. 63:1147-1154. https://pubmed.ncbi.nlm.nih.gov/18339361/
- ↑ Pryce, C.R., Rüedi-Bettschen, D., Dettling, A.C., et al. (2005) Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neuroscience and Biobehavioral Reviews. 29:649-674. https://pubmed.ncbi.nlm.nih.gov/15925698/
- ↑ 60.0 60.1 Faravelli, C., Lo Sauro, C., Lelli, L., et al. (2012) The Role of Life Events and HPA Axis in Anxiety Disorders: A Review. Current Pharmaceutical Design. 18(35):5663-5674. https://pubmed.ncbi.nlm.nih.gov/22632471/
- ↑ Bremner, J.D., Vythilingam, M., Vermetten, E., et al. (2003) MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. American Journal of Psychiatry. 160:924-32. https://pubmed.ncbi.nlm.nih.gov/12727697/
- ↑ Tarullo, A.R. and Gunnar, M.R. (2006) Child maltreatment and the developing HPA axis. Hormones and Behavior. 50:632-639. https://pubmed.ncbi.nlm.nih.gov/16876168/
- ↑ Chen, Y.H., Bai, J., Wu, D., et al. (2019) Association between fecal microbiota and generalized anxiety disorder: severity and early treatment response. Journal of Affective Disorders. 259:56–66. https://pubmed.ncbi.nlm.nih.gov/31437702/
- ↑ Whitehead, W.E., Palsson, O., and Jones, K.R. (2002) Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 122(4): 1140–1156. https://pubmed.ncbi.nlm.nih.gov/11910364/
- ↑ Amy Myers MD. Vagal Tone: The Gut-Brain Axis & the Vagus Nerve [online]. Available from: https://www.amymyersmd.com/article/vagal-tone [accessed 14 April 2024].
- ↑ Ben-Menachem, E., Hamberger, A., Hedner, T., et al. (1995) Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Research. 20:221-227. https://pubmed.ncbi.nlm.nih.gov/7796794/
- ↑ 67.0 67.1 Chae, J.H., Nahas, Z., Lomarev, M., et al. (2003) A review of functional neuroimaging studies of vagus nerve stimulation (VNS). Journal of Psychiatric Research. 37(6):443-455. https://pubmed.ncbi.nlm.nih.gov/14563375/
- ↑ Suarez, E.C., Krishnan, R.R., and Lewis, J.G. (2003) The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosomatic Medicine. 65:362–368. https://pubmed.ncbi.nlm.nih.gov/12764208/
- ↑ Felger, J.C. and Lotrich, F.E. (2013) Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 246:199–229. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3741070/
- ↑ Rush, A.J., George, M.S., Sackeim, H.A., et al. (2000) Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biological Psychiatry. 47(4):276-286. https://pubmed.ncbi.nlm.nih.gov/10686262/
- ↑ Pfuntner, A., Wier, L.M., and Stocks, C. (2013). Most Frequent Conditions in U.S. Hospitals, 2011. In: HCUP Statistical Brief #162. Agency for Healthcare Research and Quality. https://pubmed.ncbi.nlm.nih.gov/24228292/
- ↑ Jiang, H.J., Barrett, M.L., and Sheng, M. (2014). Characteristics of Hospital Stays for Nonelderly Medicaid Super-Utilizers, 2012. In: HCUP Statistical Brief #184. Agency for Healthcare Research and Quality. https://www.ncbi.nlm.nih.gov/books/NBK269028/