The Novel Microbiome of Centenarians
By Liz DeProspo
Introduction
Centenarians, or individuals over 100 years of age, exhibit relatively low susceptibility to inflammation-linked illness such as heart disease, arthritis, and certain cancers [1]. A variety of factors contribute to this longevity, including a lack of genetically inherited disorders and beneficial diet and exercise choices earlier in life. Exercise and nutrition –- as well as specific genetic differences, such as the presence of obesity-associated genes –- have been linked with ultimately determining an individual’s lifespan [2]. Beyond these well-documented contributors to overall health, however, another key biological factor has recently been examined as a contributor to the medical resiliency of these long-lived individuals: their unique microbial gut composition. While aging is generally associated with a reduction in microbial diversity and a reduced ability to fight infection[3], analysis of the gut microbiome of centenarians has revealed specific trends in the relative abundance of consequential microbial species compared to the general population.
Due to developments in healthcare and quality of living, centenarians represent the most rapidly expanding age group in many regions, and are projected to exceed 3.5 million in number globally by 2050 [3]. Because their long lives are generally free of chronic ailments and severe medical conditions, centenarians often use fewer medical resources and incur fewer medical expenses compared to their counterparts who die an earlier death[3] – making the study of centenarian microbiome features beneficial to not only individuals who wish to extend their lifespan, but to those who wish to reduce elderly contributions to hospital patient volume and insurance claims. Consequently, the unique features of centenarian microbiomes, as well as the gut changes that accompany aging and the time period that precedes death – have been recent areas of research, especially for the potential of artificially extending longevity through probiotic intervention[4]. As the ability to track gut microbiome composition over time expands, the potential for tracking the intersection of cultural and environmental features and their subsequent impacts on lifespan and the gut microbiota expands as well.
The Gut Microbiome and Immune Implications
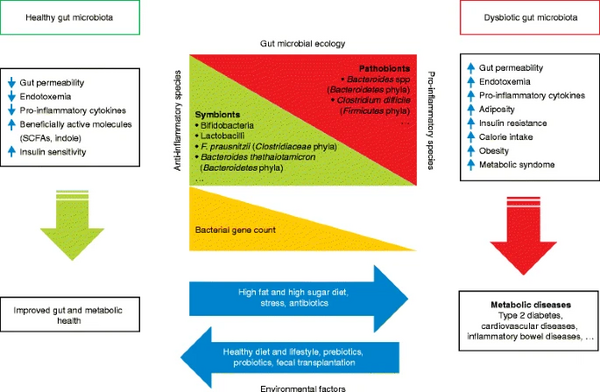
The human gut microbiome features a balance of both pathogenic and symbiotic bacteria -- many of which perform critical daily functions in maintaining the body's metabolism and homeostatic ability. The shaping of this microbiome begins in infancy and embryonic development, when maternal nutrition, mode of delivery, and infant diet introduce several key flora [2]. As an individual ages, chemicals introduced from diet, as well as repeated or improper use of antibiotics, have the potential to kill biome-balancing species and cause immune disruptions[3]. With the gut microbiome being linked with proper metabolism, immunity, and processes such as learning and memory retention, microbial gut balance is heavily consequential in an individual’s overall health [5][2].
The gut microbiome plays a vital role in the immune response by nature of regulating a range of immune-associated elements. Namely, gut microbes are instrumental in producing B and T-cells, mucosal cells, and antimicrobial proteins including lectins and cathelicidins [6]. Bacteria accomplish a range of longevity-promoting tasks within the context of the gut biome; for example, gut microbes have the capacity to mitigate fluctuations in the body's insulin response, produce molecules such as fatty acids that moderate energy metabolism, and disrupt the production of inflammatory and potentially carcinogenic cytokines [2]. In the realm of immune support, metabolic products of gut bacteria -- including these antimicrobial proteins -- are crucial components of a lytic defense against pathogenic bacteria [6].
Generally, humans have a mixture of dominant bacterial families -- Lachnospiraceae, Bacteroidaceae -- etc, as well as a variety of other viral and bacterial organisms that constitute a highly individual biome [7]. Deviations from the norm can result in health conditions associated with fluctuations or proportional shifts within the gut microbiome. For example, obesity is linked with a reduction of Bacteroides and an upregulation of Firmicutes; this causes an inflammatory response in host cells and can result in the development of metabolic syndrome [2]. Furthermore, different strains belonging to the same bacterial family can have differing effects: while Bacteroides are prevalent within the human gut microbiome, certain strains are linked with oxidative stress within the body and several health conditions, including diabetes and insulin resistance [2].
In any study of the gut microbiome and its health implications, the highly individualistic nature of a person’s gut, immune, and environmental history ensures that there is not one standard model of a healthy gut. Certain bacteria strains may prove highly beneficial in certain environmental or dietary conditions and neutral in impact in others – for example, in those living in regions where diets are often high in carbohydrates, specific strains of bacteria are more beneficial in effectively promoting carbohydrate digestion[2]. Furthermore, those who are not genetically predisposed to gastrointestinal and immune conditions may not benefit from an increased presence of specific strains via probiotics. A highly individualized blend of genetic, epigenetic, and other factors contribute to an individual’s microbiome and longevity – however, the preliminary study of especially long-lived individuals highlights several unique species that undergo changes in abundance.
Consequential Species
The changing species composition that accompanies aging does not follow a linear trajectory; while people tend to maintain a relatively stable gut microbiome from ages 20 to 60, then experience a decrease in diversity, centenarians have adapted a novel composition that is not consistent with a typical aging-related diversity reduction[3]. While certain species–namely Ruminococcaceae, Lachnospiraceae, and Bacteroidaceae, do undergo reductions in centenarians, individuals over 100 generally display an increased presence of 3 specific consequential species: Akkermansia, Bifidobacterium, and Christensenellaceae[7]. Although the specific mechanisms that underly these composition changes are still under-studied and ill-defined, researchers have posed that the recomposition of bacterial species in centenarians represents adaption to their biological conditions in old age; however, with a lack of data regarding centenarians's microbiome in their youth, it is difficult to determine whether centenarians experienced a sudden species recomposition with age, or whether these species were always present in different quantities than their short-lived counterparts[2].
Akkermansia
Akkermansia is a gram-negative Verrucomicrobia bacteria which is capable of anaerobic respiration and is frequently involved in the process of mucus degradation [8]. It is capable of secreting an enzyme that downregulates specific inflammasomes, and creates metabolic products that contribute to the production of short chain fatty acids (SCFAs) – which aid in reducing inflammation, and are linked with a decreased presence of diabetes and irritable bowel syndrome [8]. Akkermansia strains also have roles in stimulating goblet cell and macrophage production -- in addition to antimicrobial products and proper insulin secretion. Specifically, it regulated pancreatic lymphocyte production in rodents that mitigated the bodily impact of diabetes[8].
Because of the specific roles it plays in the body (and those the subsequent consequences of those roles for diseases such as diabetes, Akkermansia is linked with longevity and decreased presence of inflammatory gut-linked ailments found in centenarians. The presence of Akkermansia is typically correlated with a lower body weight (a trait common to centenarians, who typically do not suffer from obesity and related ailments), and studies involving the introduction of Akkermansia as a probiotic have shown potential in weight mitigation and diabetes prevention[8].
Bifidobacterium
Bifidobacteria is a gram-negative bacterium thought to be consequential in several gut linked health conditions -- namely, asthma and allergic disease [9]. While an overall increased presence of Bifidobacteria is present in centenarians, there are regional nuances regarding the most abundant strain; for example, while B. longum is the dominant strain of Bifidobacteria amongst Italian centenarians, B. dentium is the prevalent strain amongst Chinese centenarians[9].
Bifidobacteria are thought to have immune stimulating potential, and are linked with decreased cholesterol due to their ability to hydrolyze bile salts[9]. They have been studied as a gut regulator of specific mental health conditions, with a reduction of conditions such as depression and anxiety when introduced with mice; furthermore, one strain, B. longum, demonstrated anti anxiety effects in subjects with stimulation of enteric gut neurons and subsequent pathways[9]. Because of neuronal links between the gut and the brain, strains of bacteria such as B. longum provide a potential pathway for probiotic or fecal transplant treatment of mental health conditions -- potentially circumventing side effects and complications of traditional medications. However, such courses of treatment are still ill-studied in humans.
Beyond its mental health implications, Bifidobacteria has also been identified as a potential mechanism in attacking tumors due to their role in stimulating an uptake of T-cell activity in rodent tumors [9]. As a species that has been identified as contributing to a wide range of immune and physiological functions, the increased presence of Bifidobacteria in centenarians may be partially contributory to the relatively low cancer rates – as well as lower rates of severe mental health conditions and their physiological consequences – in centenarians.
Christensenellaceae
Christensenellacae is a Firmicute bacteria -- the presence of which is considered highly genetic in nature, with an estimated 30-60% heritability amongst humans. Christensenellacae has been linked with decreased risk of Chrohn's disease and decreased risk of high visceral fat (a type of fat that heavily contributes cardiovascular health conditions)[10]. Additionally, adults diagnosed with colorectal cancer (globally, one of the most common types of cancers) exhibit decreased Christensenellaceae presence, although the specific biological mechanisms underlying the bacterial mitigation of this cancer are currently ill-understood and highly dependent on the type of mutation present[10].
Bacterium such as Christensenellacae, with a highly heritable component, may be contributory factors for centenarian “clusters” – i.e., why those with centenarian and long-lived relatives tend to have a higher chance of reaching the same advanced age. Christensenellacae has also been shown to be highly responsive to higher levels of dietary fiber and protein – proliferating in response to beneficial dietary increases of these nutrients and subsequently aiding individuals in maintaining a lean body mass[10].
Clostridium scindens
One general area in which centenarians differ from the general population is bile acid synthesis and secondary metabolite presence. While many pathways of bile acid catabolism yield toxic byproducts, species found in centenarians are capable of synthesizing beneficial products linked with an increase in immune defense, Specifically, they harbor strains of bacteria effective at synthesizing isoalloLCA, a secondary bile acid associated with gram-positive pathogen defense and boosted immune ability [11].
One such bacterium is Clostridium scindens. Beyond synthesis of secondary metabolites that aid in a general increased immune response, Clostridium scindens is novel in its ability to convert inflammatory glucocorticoids such as cortisol to sex hormones[11]. It accomplishes this by operon-induced synthesis of an enzyme capable of cleaving cortisol's side chain in aerobic condition, a relatively rare function for gut bacteria. This specific bacteria demonstrates a link between not only the novel gut microbiome of centenarians and the immune system, but between the microbial features and regulation of bile acid, stress-induced metabolic products, and hormones within the body.
Probiotic Upregulation of Healthy Aging
Supplementary probiotic intake has been linked with reducing a variety of deleterious conditions linked with aging and early death. However, because longevity studies involving probiotics require an extensive timeframe and continual probiotic supplementation, data is both limited and difficult to achieve regarding which specific strains would be effective in extending one’s lifespan. Even so, the study of bacterial components of fermented foods and local longevity in areas where such foods are consumed can provide some insight as to the potential dietary regulation of beneficial gut bacteria[4]. So far, in studies taking place over months or years, introduction of specific probiotic supplements and the balancing of an individual’s microbial fingerprint have been associated with a decrease in insulin resistance, a decrease in the abnormal cells present in individuals with colon cancer, and other beneficial health impacts[8].
Prebiotic fermentation within the gut microbiome consists of complex relationships between several species of bacteria, with products synthesized by one species often acting as substrates for other species. Although metabolism of proteins can result in toxic byproducts, many strains of bacteria are capable of degrading such byproducts and minimizing health impacts on host organisms [1].
Certain bacteria--such as B. subtilis--are being examined as potential pathways for extending human lifespans. In several invertebrate species, introduction of B. subtilis has allowed for the formation of life-extending metabolic structures. Namely, it forms a biofilm that produces several molecules associated with anti-aging activity and reduces insulin receptor activity [4]. These molecules trigger a pathway which eventually results in the upregulation of several longevity linked transcription factors, including DAF-16 and HSF[4]. While current studies involving B. subtilis are largely confined to worms and flies, links between longevity clusters in areas wher B. subtilis is consumed (via natto in Japan, for example), suggest that longer studies involving humans will yield promising results[4].
Research has also linked the presence of specific probiotic species with improved memory retention and decreased stress on the brain; however, presently, these studies are largely confined to rodents. The species examined in these studies were primarily linked with dietary probiotic consumption, rather than a genetic or heritable bacteria difference resulting from varying gene transcription. For example, rodent exposure to Lactobacillus, a species present in a variety of milk based products, resulted in longer lifespan and decreased levels of neurological disorders analogous to dementia in humans [5].
Difficulties in Studying the Centenarian Microbiome
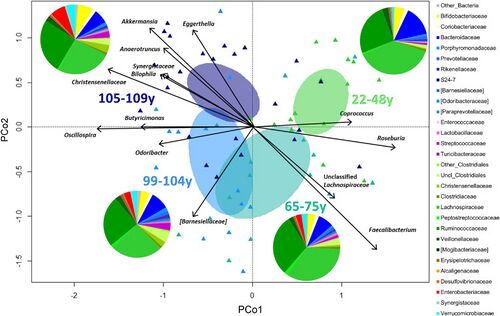
Because of vastly different lifestyle elements between different geographic regions, it can be difficult to distinguish between consequential commonalities present within the centenarian microbiome and the results of typical regional nutrition, exercise, and chemical presence. To account for this and identify the aforementioned common species that experience increases, researchers took the results of several studies done across multiple geographic areas–namely, Japan, Italy, and China–and examined which bacteria common to all centenarians were effective in separating the gut fingerprint of centenarians from their local younger counterparts [3].
In different locations, different metabolic elements were linked with varying levels of longevity. For example, while Chinese citizens displayed a direct correlation between high bile acid levels and longer lifespan, Italian elders displayed the inverse [3]. Discrepancies such as these, in addition to evolving definitions of an “elderly” individual, make it difficult to categorize and identify commonalities and predictive factors of healthy aging. Furthermore, with evolving processes such as global warming, pollution, and the increased consumption of genetically modified food products in modern countries, previous studies concerning centenarians reflect individuals who underwent nutritional and environmental conditions incredibly dissimilar from the average individual in countries such as the United States[12]; consequently, the linked between pollution and processed food and the degree of impact they have on microbiome balance and number of centenarians is a potential area of future study with the ability to inform regulatory policy regarding chemical usage in food and household products.
Microbial Changes Prior to Death
When centenarians do eventually die, the microbial changes in their gut microbiome prior to death can provide an indication of when their physical decline began. In these relatively ailment-free elders, the period preceding death is often marked by sharp and rapid decline over the course of weeks and months, rather than an extended period of suffering; this decline is reflected by the simultaneous decline of several beneficial species of bacteria[12].
In a study of Hainan centenarians, researchers cataloged a reduction in ten key bacterial species that accompanied the three months preceding when individuals eventually did die. This period of time, which typically was accompanied by a sudden decline in food intake and physical capabilities, was accompanied by the reduction of several strains -- including alisipes, bacteroides, and bifidobacterium[12]. When charted for a year prior to their deaths, these changes began their downward trajectory around seven months prior to death and often preceded the onset of noticeable symptoms[12].
However, the results of this study are highly limited in scope, as it focused on a single region with a limited sample size of 75 centenarians. The rapid decline following a seven month premortam inflection point in bacterial species abundance could also be reflective of general cultural and medical care practices involving end of life, and could differ between regions with differing end-of-life care values.
Implications for the Future
The longevity of centenarians is linked with a variety of unique microbial gut features – namely, while certain key species are found in increased presence in the centenarian gut microbiome, others are reduced. The longevity-supporting composition of their gut microbiome is likely shaped by an optimal blend of genes, environmental circumstances, diet, and lifestyle choices; however, the centenarian microbiome offers insight as to beneficial dietary choices (such as supporting beneficial bacterial growth through high protein/fiber choices and reducing sugar), as well as promising probiotics.
The use of probiotic intervention that models the centenarian dominant species composition may be an effective course of action in treating conditions such as obesity and diabetes, and increasing the overall lifespan of individuals. While it is impossible to perfectly identify or emulate the circumstances that contribute to centenarian’s longevity, probiotic supplementation or dietary support of important bacterial strains may be a first step in extending an individual’s lifespan through microbial intervention.
References
[1]
[3]
[9]
[8]
[10]
[11]
[6]
[4]
[12]
[7]
[2]
[5]
- ↑ 1.0 1.1 1.2 [Sato, Y., Atarashi, K., Plichta, D.R. et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 599, 458–464 (2021). https://doi.org/10.1038/s41586-021-03832-5]
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 [Boulangé, C.L., Neves, A.L., Chilloux, J. et al. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 8, 42 (2016). https://doi.org/10.1186/s13073-016-0303-2]
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 [Santoro A, Ostan R, Candela M, Biagi E, Brigidi P, Capri M, Franceschi C. Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci. 2018 Jan;75(1):129-148. doi: 10.1007/s00018-017-2674-y. Epub 2017 Oct 14. PMID: 29032502; PMCID: PMC5752746.]
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 [Ayala FR, Bauman C, Cogliati S, Leñini C, Bartolini M, Grau R. Microbial flora, probiotics, Bacillus subtilis and the search for a long and healthy human longevity. Microb Cell. 2017 Mar 16;4(4):133-136. doi: 10.15698/mic2017.04.569. PMID: 28435840; PMCID: PMC5376353.]
- ↑ 5.0 5.1 5.2 [Lin, S., Tsai, Y., Chen, Y., Wang, M., Chen, C., Lin, W., & Fang, T.J. (2021). Lactobacillus plantarum GKM3 Promotes Longevity, Memory Retention, and Reduces Brain Oxidation Stress in SAMP8 Mice. Nutrients, 13.]
- ↑ 6.0 6.1 6.2 [Muniz LR, Knosp C, Yeretssian G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front Immunol. 2012 Oct 9;3:310. doi: 10.3389/fimmu.2012.00310. PMID: 23087688; PMCID: PMC3466489.]
- ↑ 7.0 7.1 7.2 [Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, Capri M, Brigidi P, Candela M. Gut Microbiota and Extreme Longevity. Curr Biol. 2016 Jun 6;26(11):1480-5. doi: 10.1016/j.cub.2016.04.016. Epub 2016 May 12. PMID: 27185560.]
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 [Rodrigues VF, Elias-Oliveira J, Pereira ÍS, Pereira JA, Barbosa SC, Machado MSG, Carlos D. Akkermansia muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front Immunol. 2022 Jul 7;13:934695. doi: 10.3389/fimmu.2022.934695. PMID: 35874661; PMCID: PMC9300896.]
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 [Arboleya S, Watkins C, Stanton C, Ross RP. Gut Bifidobacteria Populations in Human Health and Aging. Front Microbiol. 2016 Aug 19;7:1204. doi: 10.3389/fmicb.2016.01204. PMID: 27594848; PMCID: PMC4990546.]
- ↑ 10.0 10.1 10.2 10.3 [Waters, J.L., Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol 17, 83 (2019). https://doi.org/10.1186/s12915-019-0699-4]
- ↑ 11.0 11.1 11.2 [Ridlon JM, Ikegawa S, Alves JM, Zhou B, Kobayashi A, Iida T, Mitamura K, Tanabe G, Serrano M, De Guzman A, Cooper P, Buck GA, Hylemon PB. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res. 2013 Sep;54(9):2437-49. doi: 10.1194/jlr.M038869. Epub 2013 Jun 15. PMID: 23772041; PMCID: PMC3735941.]
- ↑ 12.0 12.1 12.2 12.3 12.4 [Luan Z, Sun G, Huang Y, Yang Y, YangR,LiC,WangT,TanD,QiS, Jun C, Wang C, Wang S, Zhao Y and Jing Y (2020) Metagenomics Study Reveals Changes in Gut Microbiota in Centenarians: A Cohort Study of Hainan Centenarians. Front. Microbiol. 11:1474. doi: 10.3389/fmicb.2020.01474]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2023, Kenyon College
